Introduction: Newer classification systems label myelodysplastic syndrome (MDS) with 10-20% blasts and acute myeloid leukemia (AML) with MDS-related changes (AML-MR) on a continuum as they share common biologic features. The prognostic implication of blast percentage is less clinically relevant particularly in diseases with high-risk molecular features such as MDS/AML with TP53 mutations. The recently introduced Molecular International Prognostic Scoring System (IPSS-M) allows for a more personalized prognosis for MDS patients (pts) than the prior revised IPSS (IPSS-R), but it is less clear if this translates to better therapeutic choices or outcomes for pts. Allogeneic bone marrow transplantation (BMT) remains the only potentially curative approach. Increasingly clinical trials of novel compounds combined with hypomethylating agents (HMAs) have not shown improved efficacy over single agent HMAs, necessitating further study of BMT approaches for durable disease control across the spectrum of disease biology. No uniform approach to the conditioning regimen and GVHD prophylaxis for this pt population has proven superior. In the current study, we describe the outcomes of BMT with post-transplant cyclophosphamide (PTCy) for patients with high-risk MDS and oligoblastic AML-MR, and analyze the prognostic implication of their chromosomal and molecular profiles within the international scoring systems.
Patients and methods: This is a retrospective analysis of patients with 5-30% blasts and IPSS-R ≥3 who underwent BMT with PTCy at Johns Hopkins Hospital between 1/2013 and 12/2020. Next generation sequencing (NGS) was performed within 12 months of diagnosis. Kaplan-Meier analysis was performed to evaluate overall survival (OS) and relapse-free survival (RFS). Competing risks analysis was performed to evaluate the incidence of relapse and non-relapse mortality (NRM). Cox-regression models were performed in univariate analyses with false discovery rate adjustment and then subsequently conducted for multivariable analyses to identify statistically significant and independent prognostic factors.
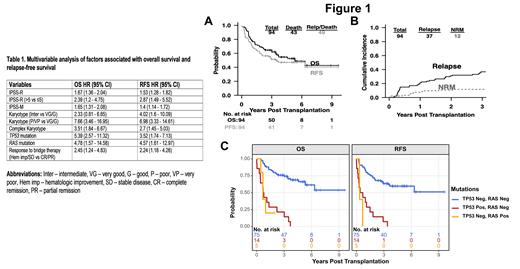
Results: Ninety-four consecutive patients (65% male) were identified with a median age of 61 (range 22 - 75) years at diagnosis. Median follow-up was 4.8 years (range 5 days-9.6 years) post BMT. Two-thirds (63%) underwent haploidentical transplantation, and 91% received reduced intensity conditioning with Fludarabine, Cyclophosphamide, and TBI. Median age at BMT was 65 years (range 22 - 76). The median time from diagnosis to BMT was 7.9 months. 52 (57%) received HMA based treatment and 36 (39%) intensive chemotherapy prior to BMT. Graft failure occurred in 6 patients (6.4%) The 5-year OS rate was 53% (95% CI: 43%-65%), the 5-year RFS rate was 47% (95% CI: 38%-59%) ( Figure 1A). The incidence of relapse was 21% (95% CI: 13% - 30%) at 1 year and 31% (95% CI: 21% - 40%) at 2 years after BMT ( Figure 1B). NRM rate in the first-year was 7% (95% CI: 2%-13%) and 11% (95% CI: 4%-17%) in the first two years ( Figure 1B). The incidence of acute GVHD (aGVHD) grades 3 to 4 was 3.2%, whereas chronic GVHD (cGVHD) presented in 12 patients (12.7%) with only 2 patients (2.1%) having moderate to severe disease based on the NIH grading criteria. Univariate analysis showed that higher IPSS-R (HR 1.68, 95% CI 1.39 - 2.04), poor or very poor karyotype risk (HR 7.48, 95% CI 3.49 - 16.02), complex karyotype (HR 3.72, 95% CI 2.01 - 6.88), higher IPSS-M (HR 1.72, 95% CI 1.38 - 2.14), TP53 mutations (HR 6.53, 95% CI 3.36 - 12.7) and RAS mutations (HR 4.96, 95% CI 1.7 - 14.5) were associated with worse OS. IPSS-R was significantly correlated with the patients' response to bridge therapy (OR 1.34, P=0.036). IPSS-R, IPSS-M, poor or very poor karyotype risk, complex karyotype, TP53 and any RAS mutations and response to bridge therapy were independently associated with worse OS and RFS ( Table 1, Figure 1C).
Conclusion: This retrospective analysis supports that BMT with PTCy is a durable approach for patients with high-risk MDS and oligoblastic AML-MR leading to high OS and RFS associated with a promising safety profile with low NRM and severe GVHD incidence. IPSS-R, IPSS-M, karyotype and response to bridge therapy are independent predictors of OS and RFS post-BMT. The presence of TP53 and/or RAS mutations defines a sub-group of patients with dismal outcomes representing an unmet need within this patient population.
Disclosures
DeZern:Geron: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Caribou: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy; Sobi: Consultancy; Appellis: Consultancy, Membership on an entity's Board of Directors or advisory committees.