Introduction
Results of the unplanned interim analysis of the ATLAS trial (Dytfeld, Lancet Oncology 2023) suggest a progression-free survival benefit of carfilzomib, lenalidomide, and dexamethasone (KRd) post-transplant maintenance over single-agent lenalidomide (R). Use of triplet combination, with frequent hospital visits and repeated intravenous infusions raises questions about its long-term tolerability and the impact on health-related quality of life (HRQoL) compared with relatively convenient and well-tolerated single-agent oral lenalidomide. Here, we present a descriptive, longitudinal assessment of HRQoL in Polish patients with multiple myeloma treated in the ATLAS trial.
Methods
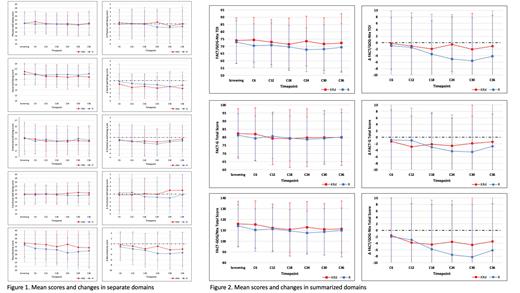
ATLAS is an ongoing phase 3 trial that randomized 180 patients with newly-diagnosed multiple myeloma after ASCT to receive maintenance therapy with KRd or R. In the KRd arm, patients with standard-risk cytogenetics who achieved MRD-negativity after cycle 6 de-escalated therapy to receive R alone after cycle 8. HRQoL assessment was not required by the study protocol, however, the FACT/GOG-Ntx questionnaires were collected according to local protocols for post-transplant data collection at 7 Polish sites at screening (approximately 3 months post-transplant) and after cycles (C) 6, 12, 18, 24, 30, and 36. Therefore, the modified intention-to-treat (mITT) population in this analysis consisted of 134 patients enrolled in these sites. The domains evaluated by the FACT/GOG-Ntx questionnaire include physical well-being (PWB), social well-being (SWB), emotional well-being (EWB), functional well-being (FWB), and neurotoxicity (Ntx). The outcomes are summarized in FACT/GOG-Ntx Trial Outcome Index (TOI, combining PWB, FWB, and Ntx), FACT-G Total Score (PWB, SWB, EWB, and FWB), and FACT/GOG-Ntx Total Score (all five domains). Based on previous reports, the minimally important difference (MID) was defined as a change of at least 10% of the instrument range (Tremblay, BMC Cancer 2021). Student's t-test was used for comparisons between normally distributed variables and Wilcoxon rank-sum test for not normally distributed. Rates of patients experiencing MID were compared using chi-square test.
Results
HRQoL questionnaires were available for 123 out of 134 patients (92%). The numbers of completed questionnaires at screening, C6, C12, C18, C24, C30, C36 were 115 (86% of randomized patients / 86% of expected patients), 111 (83%/86%), 102 (76%/85%), 93 (69%/85%), 82 (61%/86%), 74 (55%/87%), and 56 (42%/80%), respectively. Throughout the analyzed timepoints, the mean PWB, SWB, EWB, FWB, NTx scores (Figure 1), FACT/GOG-NTx TOI, FACT-G Total Score, and FACT/GOG-Ntx Total Score (Figure 2) did not differ between the two study arms. A similar pattern was observed in the changes in individual patient scores compared to baseline. During the treatment course, the rates of patients experiencing a decrease in HRQoL scores, reaching the MID threshold in at least one evaluation, were consistent in both groups - 19 out of 59 (32%) patients treated with KRd and 20 out of 52 (38%) patients in the R arm (p=0.49) in the FACT/GOG-NTx TOI, 23/59 (39%) in the KRd arm and 21/52 (40%) in the R arm in the FACT-G Total Score (p=0.88), and 24/59 (41%) in the KRd arm and 19/52 (37%) in the R arm in the FACT/GOG-Ntx Total Score (p=0.65). Similarly, there were no differences in the respective results in terms of patients experiencing an increase in HRQoL scores, reaching the MID threshold in at least one evaluation - 8 out of 59 (14%) patients treated with KRd and 11 out of 52 (21%) patients in the R arm (p=0.28) in the FACT/GOG-NTx TOI, 10/59 (17%) in the KRd arm and 13/52 (25%) in the R arm in the FACT-G Total Score (p=0.29), and 8/59 (14%) in the KRd arm and 6/52 (12%) in the R arm in the FACT/GOG-Ntx Total Score (p=0.75).
Conclusions
The results of this analysis do not imply a negative impact of post-transplant maintenance with carfilzomib, lenalidomide, and dexamethasone compared to single-agent lenalidomide on the HRQoL.
OffLabel Disclosure:
Derman:Janssen: Consultancy, Honoraria; COTA Healthcare: Consultancy. Gil:Celgene/BMS: Honoraria; Janssen: Honoraria; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria; Astellas: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Zaucha:Medical University of Gdańsk: Current Employment; BMS: Research Funding; Pierre Fabre, Takeda, BMS, Gilead, Novartis, Pfizer, Amgen, F. Hoffmann-La Roche Ltd, Astra Zeneca, Abbvie: Honoraria; MSD: Research Funding. Walewski:Amgen: Honoraria; Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; GSK/Novartis: Research Funding; Servier: Honoraria; Abbvie: Consultancy, Honoraria. Robak:Janssen: Consultancy, Honoraria, Research Funding; Abbvie: Honoraria; OctoPharma: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; GSK: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Regeneron: Honoraria, Research Funding. Kruk-Kwapisz:Clinscience: Current Employment. Jakubowiak:Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi-Aventi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Dytfeld:BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Carfilzomib, Lenalidomide, dexamethasone combination as maintenance therapy post autologous stem cell transplantation in multiple myeloma.