INTRODUCTION
Lenalidomide (len) maintenance has been considered the standard of care treatment following an autologous stem cell transplantation(autoSCT) for patients (pts) with newly diagnosed multiple myeloma (MM). Len doses of up to 15 mg are used in this setting, however in clinical practice patients may experience a variety of side effects including neutropenia, infections, diarrhea, rash, or secondary malignancies, which may impact the quality of life and potentially lead to discontinuation of therapy.
In this retrospective analysis, we aim to evaluate the effects of len dose reductions on progression-free survival (PFS), overall survival (OS), and other outcome measures in a post-autoSCT maintenance setting using the multiple myeloma database at our center.
METHODS
We reviewed patients' clinical and demographic characteristics including age and R-ISS stage at diagnosis and IMWG response after autoSCT. We included patients diagnosed with MM between 01/01/2010 to 02/28/2022. We compared pts receiving a full dose (15 mg/day) of len (control group) versus those requiring dose reductions or receiving lower doses to begin with (dose reduction group). The primary end-point was PFS, secondary end-points included OS, reasons for dose reduction, correlation with initial and follow-up disease parameters, and long-term toxicities. Demographic and clinical characteristics were summarized by cohort and compared using Mann-Whitney U and Fisher's exact tests. The PFS at (6 months and one year) and OS were summarized by cohort using Kaplan-Meier methods. Multivariate Cox regression analysis was completed adjusting for age, stage at diagnosis, prior response, full versus reduced len doses, and duration of treatment. Analyses were performed using SAS v9.4 at a significance level of 0.05.
RESULTS
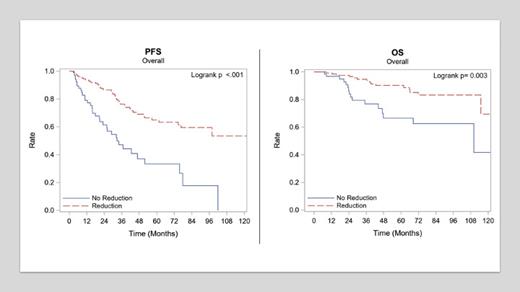
The mean age at diagnosis was 61 and matched between groups. The median follow-up time in months was 52.9 months (range: 2.8-140.2 months). A total of 66% (134/203) of patients had lower doses than 15mg, while 34% (69/203) were maintained at 15mg of len. For the control group, 20% (n=14) had RISS I, 48% (n=33) II, and 13% (n=9) III, at the time of diagnosis, respectively; for the dose reduction group, and 38% (n=37) stage I, 39% (n=51) stage II, and 9% (n=12) stage III (remainder unknown) for the control group. These differences were not statistically significant (p=0.35). In the control group, 49% (n=33) pts had achieved CR prior to initiation of maintenance compared to 65% (n = 86) in the dose reduction group which was borderline statistically significantly different (p=0.06). Achieving CR before maintenance before maintenance was prognostically beneficial (p<0.001). The most common reason for discontinuation of len, was progression in both groups (control group 84% (n=31), dose reduction group. 41% (n=29)). This was however still statistically significantly different between the 2 groups (p<0.01). The most common reasons for dose reductions in the respective group were neutropenia (56.8%, n= 75), neuropathy (12%, n=15), and rash (9%, n=12). The duration of treatment in the control group and the dose reduction group was 24 months and 30 months respectively (p=0.113). Surprisingly, there was a statistically significant better PFS (p<0.001) and OS (p=0.003) for patients who received len at a reduced dose (figure 1 and 2). However, when adjusted for prior response using Multivariate cox regression analysis, there was no statistically significant difference in outcome between the 2 groups.
CONCLUSION
This retrospective analysis shows that dose reduction of len did not lead to worse PFS or OS, especially in those who achieved CR prior to maintenance. According to our data for those who fail to achieve a CR post autoSCT, increasing the dose of len to 15 mg (standard dosing practice) may lead to increased toxicities while failing to affect PFS or OS significantly.
A potential bias towards long OS findings may be that patients in this investigation were limited to those who were able to get to and actually receive autoSCT. They were also not felt to require more intense maintenance with more than 1 drug. Based on real-world experience and prior studies looking at various dose schedules of len, we theorized that dose reduction may not have a large negative impact on long-term clinical outcomes.
Disclosures
Hillengass:Janssen: Consultancy, Other: DSMB; Prothena: Consultancy; Amgen: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Beigene: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; ESMO Florida: Other: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Targeted Oncology: Other: Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events; Angitia: Consultancy; OncLive: Consultancy; Axxess Network: Consultancy; Sanofi: Consultancy; Oncopeptides: Consultancy; Skyline: Consultancy; GSK: Consultancy, Research Funding.