Chimeric antigen receptor (CAR) T cells have been proven as a highly promising immunotherapy for patients with multiple myeloma (MM). Apheresis of autologous CD3+ lymphocytes is the first pivotal step in the production process of chimeric antigen receptor (CAR) T cells. Repetitive activation of T lymphocytes can affect the qualitative properties, i.e. the fitness of CD3 cells leading to progressive loss of expression of the costimulatory receptors CD27 and CD28. CD3+27-28- cells are non-proliferating memory, senescent cells. In that regard, the factors influencing the manufacturing process of CAR T cells in patients with MM have not been sufficiently explored.
We performed a retrospective analysis of clinical parameters influencing both collection of autologous lymphocytes and the subsequent manufacturing of idecabtagene-vicleucel with a special focus on T cell senescence, defined as loss of CD27 and CD28. Furthermore, we focused on cell-compositions of collections resulting in out of specification (OOS) events during CAR T cell manufacturing.
Between January 2022 and January 2023, 36 lymphocyte collections of 32 patients with relapsed/refractory (r/r) MM were performed for subsequent CAR T cell therapy with idecabtagene-vicleucel. All collections were forwarded for further manufacturing without prior cryokonservation. 9 (25%) collections resulted in OOS; 7/9 resulting in insufficient proliferation or expansion of T cells, while 2/9 showed a microbial contamination during the manufacturing process after viral transduction for CAR generation.
At apheresis, the patients were a median 63 (range 39-75) years old, 12 (38%) were female with median body weight 79 kg (49-103). The patients had a median of 7 previous treatment lines (range 3-13) and 15/36 collections were performed at partial remission or better. 8/36 collection had prior exposition to bispecific antibodies whereas in 13/36 collections the unspecific positivity for cytomegalovirus (CMV) in polymerase chain reaction (PCR) was detected. The collections were performed on a Spectra Optia cell-separator by processing median 3x total blood volume.
CD3+ cells in peripheral blood (pB) were median 513.9 /µl (range 70.6-3616.0).
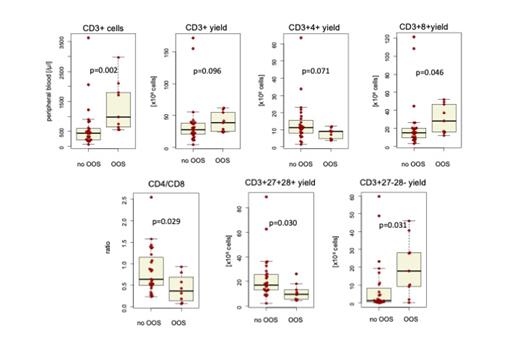
The collections showed a median CD3+ yield with 29.8 x10 8 (range 4.9-171.6), with CD3+4+ and CD3+8+ yields 10.6 x10 8 (range 1-663.4) and 16.3 x10 8 (range 3.2-121.2) respectively, resulting in median CD4:CD8 ratio of 0.6 (range 0.1-2.6).
The median yields for CD3+27+28+ and CD3+27-28- cells were 14.8 (range 2.3-88.8) x10 8 and 2.1 (range 0.1-59.8) x10 8 respectively.
Noteworthy, OOS events were more common in patients with detectable cytomegalovirus replication at apheresis ( P=0.037) with a trend for prior exposition to bispecific antibodies ( P=0.086). In contrast, the collections with OOS did not differ regarding patients' age ( P=0.498), sex ( P=0.442), body weight ( P=0.364), number of previous treatment lines ( P=0.185) or remission status at apheresis ( P=0.443).
In OOS collections, CD3+ cells in pB were significantly higher with median 991 /µl (range 558-2968) compared to 448.9 /µl (70.6-3616.0) ( P=0.002) and showed a trend for higher CD3+ yields with 38.9 x10 8 (range 23.9-62.2) and 28.2 x10 8 (range 4.9-171.6) respectively ( P=0.096).
Interestingly, OOS products also had higher median yields of CD3+CD8+ cells with 28.1 x10 8 (range 12.2-52.3) compared to 15.3 x10 8 (range 3.2-121.2), ( P=0.046) and showed a trend for lower median CD3+CD4+ yields with 9.1 x10 8 (range 3.8-11.9) compared to 11.4 x10 8 (range 1.6-63.4), ( P=0.071).
Median CD3+27+28+ yields in OOS and successful manufacturings were 9.7 x10 8 (4.7-26.0) and 17.0 x10 8(range 2.4-88.8) respectively ( P=0.030). OOS events yielded with higher median CD3+27-28- with 17.8 x10 8(range 0.2-46.1) compared to 1.4 x10 8 (0.1-59.8), ( P=0.031).
We observed a negative prognostic role of CMV replication as well as of senescence of
T lymphocytes on the manufacturing process. In contrast, a successful manufacturing was not influenced by patient age, sex, remission status at apheresis or number of previous treatment lines. Further analyses are required to determine measures appropriate for further optimization of autologous lymphocyte collections and improvement of collection outcomes in patients with MM.
Disclosures
Vucinic:Abbvie: Honoraria; Takeda: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Sobi: Honoraria, Other: Travel/Accommodations/Expenses; Amgen: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; Gilead/Kite: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria, Other: Travel/Accommodations/Expenses; Novartis: Consultancy, Honoraria. Heyn:Janssen: Honoraria. Kloetzer:Janssen: Honoraria. Herling:Mundipharma EDO, Janpix, Novartis, Roche: Research Funding; Abbvie, Beigene, Janssen, Stemline, Takeda: Consultancy. Jentzsch:Novartis: Honoraria; Amgen: Honoraria; Blueprint Medicine: Honoraria; Pfizer: Honoraria; BMS: Honoraria; JAZZ: Honoraria. Platzbecker:Janssen Biotech: Consultancy, Research Funding; Syros: Consultancy, Honoraria, Research Funding; Merck: Research Funding; Jazz: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Celgene: Honoraria; AbbVie: Consultancy; Curis: Consultancy, Research Funding; MDS Foundation: Membership on an entity's Board of Directors or advisory committees; Silence Therapeutics: Consultancy, Honoraria, Research Funding; Servier: Consultancy, Honoraria, Research Funding; Geron: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; medical writing support, Research Funding; Amgen: Consultancy, Research Funding; Fibrogen: Research Funding; Roche: Research Funding; BeiGene: Research Funding; BMS: Research Funding. Merz:AMGEN, TAKEDA, BMS, JANSSEN, STEMLINE, ROCHE: Honoraria.