BACKGROUND: In the context of the TT4 study, a phase 3 trial was conducted to investigate whether a modified fixed duration treatment approach, known as L-TT4, could reduce toxicity while maintaining efficacy in tandem transplants for low-risk multiple myeloma (MM) defined by gene expression profiling. Here we report final analysis of TT4 study with long-term follow up of 11.7 years.
METHODS: Treatment modification involved reducing the number of induction and consolidation cycles from 2 cycles each in the standard S-TT4 regimen to 1 cycle in L-TT4. The transplant regimen in L-TT4 was further adjusted by administering melphalan in a fractionated schedule of 50mg/m2 per day for 4 days (MEL50x4), instead of a single high-dose (MEL200) to reduce toxicity. To compensate for the potential decrease in effectiveness, bortezomib, thalidomide and dexamethasone (VTD) added for synergy between MEL50x4 and VTD. Maintenance was with 3 years of bortezomib, lenalidomide and dexamethasone The goal of this trial was to evaluate the impact of these modifications on toxicity levels and treatment outcomes in patients undergoing tandem transplants for multiple myeloma. Patients were enrolled in the period of August 2008 until March 2016. Data cut-off was July,2023.
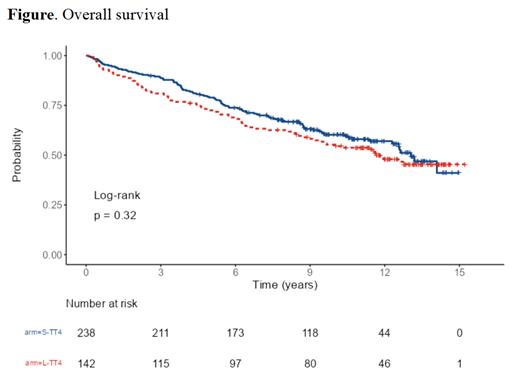
Results: Our study consisted of 380 patients after excluding 2 patients who were identified as high-risk gene expression profile (S-TT4:238, L-TT4:142). Median age was 58.7 years (range: 30-76 years) with 11.6% of enrolled patients identified as African Americans. 91.4% of enrolled patients completed at least one auto transplant and 78.3% of patient completed tandem transplants. The median follow-up duration for all patients of 11.66 years (interquartile range: 10.11-13.27 years). Median progression free survival (PFS) was 6.54 years (95% confidence interval (CI): 5.75-7.72 years)) with 10-year PFS of 39% (95% CI: 34.3-44.4%). Median overall survival (OS) was 12.6 years (95% CI: 11.6 years-not reached (NR)) with 10-year OS of 58.4% (95% CI:53.5%-63.7%). No statistically significant difference between S-TT4 vs. L-TT4 (median PFS: 7.33 years (95% CI: 6.01-9.44 years), median OS: 13.1 years (95% CI:12.30 years-NR) vs. (median PFS: 5.74 years (95% CI: 4.63-7.41 years),median OS:11.8 years (95% CI:9.21 years-NR) with OS hazard ratio of L-TT4 vs. S-TT4 of 1.17 (95% CI: 0.86-1.58, p=0322). [Figure] PFS HR of L-TT4 vs. S-TT4 of 1.21 (95% CI: 0.94-1.56, P=0.132). Early mortality (defined as death in the first year of enrollment) occurred in 6.1% of enrolled patients. No difference in treatment related mortality was found between L-TT4 and S-TT4. Grade III-V toxicities and maximum grade for system organ classes with at least 5% of the population experiencing an event showed no difference between L-TT4 vs. S-TT4 in all adverse events except grade III/IV infections which was higher in L-TT4 (60% vs. 44%, p=0.002). Secondary acute myeloid leukemia (AML) and/or myelodysplastic syndrome (MDS) occurred in 21 (5.5%) patients with the median time from diagnosis to diagnosis of AML and/or MDS of 4.5 years. AML and/or MDS was more frequently seen in patients assigned to L-TT4 when compared to S-TT4 though this difference was not statistically significant (7% vs. 4.6, P=0.317 )
Conclusion: Fixed duration therapy including multi-agent chemotherapy and tandem autologous stem cell transplantation and 3 years of extended therapy resulted in excellent patients' outcomes with median OS of 12.6 years and at least one in three patients being progression free for at least 10 years. Conditioning with single high-dose (MEL200) as used in S-TT4 resulted in similar outcomes compared to fractionated MEL50x4 combined with VTD with better safety profile and thus should be the preferred approach.
Disclosures
van Rhee:GlaxoSmithKline: Consultancy; EUSA Bio: Consultancy; Adicet Bio: Consultancy; Bristol Myers Squibb: Research Funding; Janssen Pharmaceuticals: Research Funding.