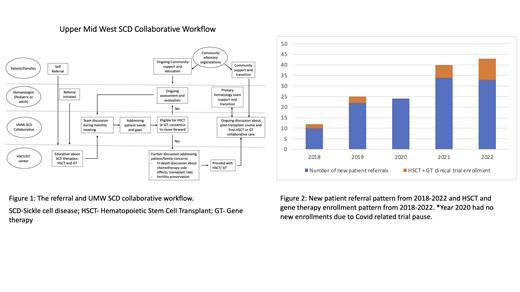
Introduction:
Sickle cell disease (SCD) is the most common inherited hemoglobinopathy affecting populations across the United States. Several well-established patient-centered comprehensive care models have been in practice for preventive care and pain management. However, access to hematopoietic stem cell transplant (HSCT) and clinical trials for sickle cell gene therapies are limited, especially in pediatric and adult centers with limited HSCT-related resources. We describe a patient-centered collaborative model in the Upper Midwest United States to improve patient education and engagement as well as access to HSCT and gene therapy trials for patients with SCD.
Collaborative Care Model:
Since August 2021, centers across the Midwest meet monthly to improve access for HSCT and gene therapy as well as education about these therapies for individuals with SCD. The teams include: hematology and transplant teams from University of Minnesota, Comprehensive Sickle Cell Care team from Children's Hospital of Minneapolis, hematology and transplant team from Mayo Children's Hospital, hematology team from Essential Health, Duluth, Minnesota, hematology team from Sanford Children's Hospital Fargo and Sioux Falls and hematology team from Blank Children's Hospital, Des Moines, Iowa. This collaborative approach involves several stakeholders including an SCD community advocacy organization, hematology care providers across several rural as well as community/university hospitals, social workers, nurse coordinators, HSCT/gene therapy providers, as well as representatives from National Marrow Donor Program. Individual patient needs and available community support as well as institutional resources are discussed at length during monthly virtual meetings and a pre-intervention plan is established to ensure smooth transition of care to and from primary centers (Figure 1). Ongoing communication with the primary team helps with efficient transition of care after transplant as patients continue ongoing monitoring and care closer to home. Community partners help with early education of the families and provide necessary resources and support. Institutional partners like NMDP provide support with immune typing and discuss ongoing support and other resources available for providers and families.
Results/Impact:
Since the implementation of this collaborative model, there has been a steady increase in early referrals for education regarding HSCT and potential gene therapies trials for patients with SCD. There has been about 100% increase in new patient referrals since the implementation of this collaborative model compared to the average new referrals in the previous years (2021-22 vs 2018-19; Figure 2). Enrollment in HSCT as well as gene therapy clinical trials also has increased 2-3 folds since the implementation of the collaborative care model. It has helped established early discussion and longitudinal care and has also led to ongoing evaluation and discussions for availability of optimal donors, determining eligibility for HSCT and gene therapy trials as well as providing ongoing support and education to the sickle cell community and providers regarding the available therapies for SCD. This has also facilitated the transplant team establish earlier and better rapport with patients and their families and provide necessary education with an in-depth discussion regarding the potential benefits and risks of therapies such as HSCT and gene therapy. These discussions often occur over multiple visits and helps address psychological as well social barriers with a prospective evaluation. Additionally, the ongoing discussion among the providers helps with longitudinal monitoring of the patients and provide an assessment of the risks and benefits of therapies as the patient's disease course continues to evolve. This multi-stake holder collaborative approach continues to provide a well-established platform for discussion of newer therapies and access to open clinical trials, available resources for patients and families, and improve patient care and experience. The impact of this collaboration is noted in significant increase in early referrals and educational consults for HSCT and gene therapies, improved patient engagement as well as the acceptability and participation in clinical trials.
Disclosures
Blaylark:Agios Pharmaceuticals: Consultancy, Honoraria. Boucher:Takeda: Research Funding; CSL Behring: Research Funding. Fritch Lilla:Chiesi: Membership on an entity's Board of Directors or advisory committees. Gupta:Bluerock Therapeutics: Membership on an entity's Board of Directors or advisory committees; Vertex Pharmaceuticals: Consultancy.