Background: The treatment a patient with AML receives may be influenced by where the patient is diagnosed and the physician making the initial diagnosis. Such factors could be targeted for intervention to help ensure patients receive optimal treatment; however, data constraints have previously limited a complete examination of patient, physician and hospital factors associated with treatment for AML. This study aimed to investigate the association of patient, hospital, and physician factors with treatment receipt for patients diagnosed with AML. Treatment receipt included allogeneic hematopoietic cell transplant (alloHCT) or chemotherapy without alloHCT among patients who received a National Marrow Donor Program (NMDP) donor search (search).
Methods: Centers for Medicare and Medicaid (CMS) Medicare administrative claims data from 2010 to 2018 were linked to NMDP Donor Search data and to publicly available CMS and National Provider Identifier physician and hospital datasets. Patients aged 65-74 diagnosed with AML between 2010 and 2017 and enrolled in fee-for-service (Part A and B) Medicare for at least one year after diagnosis or until death, were included. The treatment groups (alloHCT and search) were identified using diagnosis and procedure codes. A multivariable hierarchical logistic regression procedure was performed to determine factors associated with treatment receipt, with hospital as a random effect. Models were adjusted for patient, hospital and physician characteristics identified at the time of diagnosis. Patients missing physician and hospital characteristics were excluded. Individuals with a racial/ethnic background of Black/African-American, Asian/Pacific Islander, Hispanic, American Indian/Alaska Native, and Other were grouped due to small numbers. All analyses were performed using Statistical Analysis Software (SAS ®) Enterprise Guide 7.1.
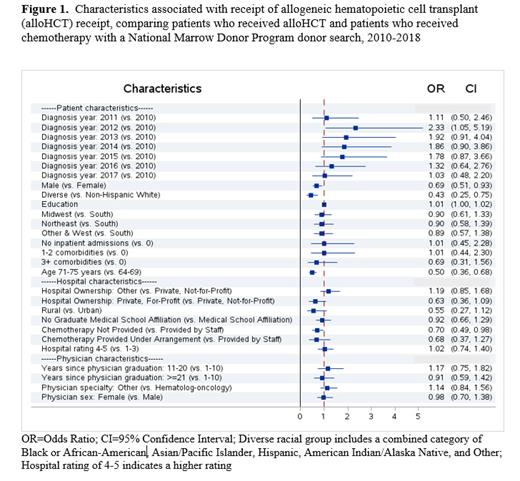
Results: A total of 916 patients met eligibility criteria: 554 (60%) received alloHCT; 362 (40%) received search. The majority of patients in both groups were Non-Hispanic White and were age 65-69 years. A multivariable model demonstrated the variables associated with receipt of alloHCT (Figure 1). Characteristics significantly associated with decreased odds of receiving alloHCT were sex (male compared to female), race/ethnicity (a combined category of Black/African-American, Asian/Pacific Islander, Hispanic, American Indian/Alaska Native, and Other compared to Non-Hispanic White), living in an area with a lower percentage of population with a college education, age (71-75 compared to 65-69 years), and chemotherapy provision (chemotherapy not compared to provided by staff).
Conclusion: This study used novel linked datasets to identify the association of patient, hospital, and physician characteristics at time of diagnosis with receipt of alloHCT, compared to patients who received a search but not an alloHCT. Notably, patient characteristics, and the hospital characteristic of provision of chemotherapy by hospital staff were associated with alloHCT receipt. Donor availability may contribute to the decreased odds of alloHCT by race/ethnicity, but studies to increase HLA-mismatched unrelated alloHCT are helping to decrease this barrier. Neighborhood education attainment was used as a proxy for socioeconomic status (SES), and SES has been associated with access to care. Ensuring treatment information is accessible and available in plain language may help patients considering different treatment options. Claims data is not collected for research, and has some inherent limitations, including the lack of disease severity, cytogenetics, patient preferences, and an algorithm was used to identify diagnosing physician and hospital. Additionally, this analysis includes patients who were able to access a transplant center to receive a search or alloHCT. Next steps will compare characteristics of those who received chemotherapy but did not receive a search to those who received a search and those who received an alloHCT. Study results will be used to help inform strategies aimed at improving access to treatment for patients diagnosed with AML.
Disclosures
Meyer:Sanofi: Research Funding.