Abstract
Influenza viral infections have the ability to reprogram the immune system of the host and alter its functions to fight infections. However, the exact mechanisms and functions of virus-induced innate immunity in the context of leukemia are not yet fully understood. Our previous research has shown that a patient with refractory acute myeloid leukemia (AML) achieved complete remission (CR) after being infected with the Influenza A virus (IAV, H1N1 subtype), and this was functionally validated in two different animal disease relevant models. Our recent study demonstrates that mice pre-trained with the IAV promotes a long-term anti-leukemia effect. The anti-leukemia activity of IAV-induced trained immunity is due to the transcriptomic rewiring of hematopoietic stem and progenitor cells (HSPCs), resulting in a sustained enhancement of myeloid cells reprogramming toward an antitumor phenotype. This mechanism involves the upregulation of type I interferons (IFNs) and subsequent downregulation of inflammatory proteins. Our findings shed light on the dynamic changes in innate immune cells following viral infections and indicate a novel anti-leukemia facet of appropriate rewiring of granulopoiesis via upregulation of type I IFNs.
Statement of significance
The study profiles the dynamic changes in immune cells during exposure to the Influenza virus and sheds light on the role of Influenza trained innate immunity in anti-leukemia effect. The study also explores a novel anti-leukemia facet of appropriate rewiring of granulopoiesis via upregulation of the type I IFN pathway. These findings suggest that innate immune training could be a promising adjuvant therapy for cancer and warrant further investigation.
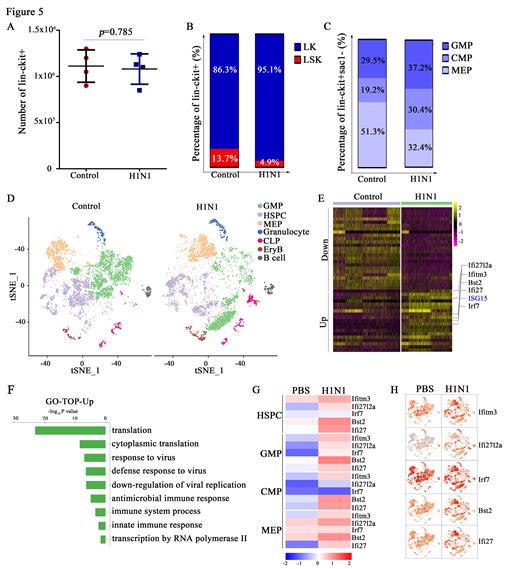
Legend for Figure 5 . H1N1 reprogrammed granulopoiesis via up-regulation of type I interferon pathway.
(A-C) FCM analyses and absolute counts of BM progenitor cells on day 7 after training with H1N1 or PBS. FCM, flow cytometry; BM, bone marrow; LK, Lin-c-kit+Sac1-; LSK, Lin-c-kit+Sac1+; GMP, granulocyte-monocyte progenitors (Lin-c-kit+Sac1-CD16/32 hiCD34+); CMP, common myeloid progenitors (Lin-c-kit+Sac1-CD16/32 intCD34+); MEP, megakaryocyte-erythroid progenitor cell (Lin-c-kit+Sac1-CD16/32-CD34-).
(D) T-SNE plot of top 7 progenitor cells population changes. t-SNE, t-distributed stochastic neighbor embedding.
(E) Heatmap visualization of the expression of different genes in HSPCs. The upregulated genes related to the type I IFNs pathway are listed on the right. HSPCs and hematopoietic stem/progenitor cells.
(F) The top enriched GO terms of biological processes for upregulated genes in H1N1 trained group. GO, gene ontology.
(G-H) Heatmap and t-SNE plot of type I IFN-associated upregulation genes in progenitor cells of HSPCs, GMP, CMP, and MEP. t-SNE, t-distributed stochastic neighbor embedding.
Disclosures
No relevant conflicts of interest to declare.