BACKGROUND
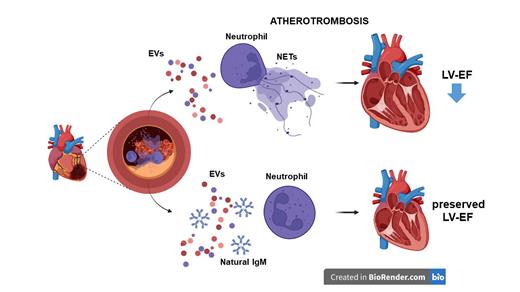
Neutrophil extracellular traps (NETs) emerged as important contributors to thrombus formation. We have previously demonstrated the role of NETs in acute myocardial infarction (AMI). However, the mechanistic understanding of modulators of NETosis in the context of AMI is scarce. Levels of extracellular vesicles (EV) carrying lipid peroxidation-derived epitopes also called oxidation-specific epitopes (OSE), are increased at the culprit site in AMI. Importantly, natural IgM antibodies with specificity for OSE have been shown to inhibit the pro-coagulatory and pro-inflammatory effects of EV, and low plasma levels predict cardiovascular risk. We hypothesized that large EV, also called microvesicles (MV), induce NETosis during AMI, and that natural anti-OSE IgM are inhibiting this process.
METHODS
Patients were consented when the decision for primary percutaneous intervention (pPCI) for acute ST-segment elevation myocardial infarction (STEMI) was made, and blood was aspirated from the culprit site and peripheral site as an in-patient systemic control (n=28). Myocardial function was assessed by cardiac magnetic resonance imaging (cMRI) 4±2 days and 195±15 days after pPCI. EV were isolated from patient culprit site plasma and cell culture supernatants and characterized by flow cytometry. Isolated EV were used for neutrophil stimulation in vitro, and in vivo using a murine injection model in the presence of the IgM LR04 recognizing the immunodominant OSE, malondialdehyde (MDA) epitopes, or an isotype control. NETs were visualized by immunofluorescence staining for DNA-histone, citrullinated histone 3 (citH3) and myeloperoxidase (MPO). NET markers and natural IgM recognizing OSE in murine and patient plasma were measured by ELISA.
RESULTS
Culprit site plasma contained more and proportionally higher levels of leukocyte-derived CD45+ MDA-EV compared to the peripheral site plasma. NET markers were associated with CD45+ MDA-EV at the site of occlusion. Decreased levels of MDA-specific IgM at the culprit site indicated consumption of protective IgM. EV isolated from AMI patient plasma induced NETosis in vitro and after injection into C57BL/6 mice in vivo, as determined by NET markers and fluorescence microscopy of histone citrullination in neutrophils. The malondialdehyde-specific IgM LR04, but not a control IgM, reduced the NETogenic effects of EV in vitro and in vivo. Higher circulating levels of EV and lower OSE-IgM were associated with reduced ejection fraction in AMI patients at follow-up.
CONCLUSION
EV from AMI patients induced NET formation in vitro and in vivo, and natural IgM recognizing malondialdehyde-epitopes attenuated this effect. In summary, the balance between OSE-EV and OSE-IgM at the culprit site during AMI may represent a potential prognostic and therapeutic target. Moreover, the modulatory effect of natural IgM may also apply to other pathologies where NETosis contributes to thrombus formation.
Disclosures
No relevant conflicts of interest to declare.