Background: Prophylaxis with von Willebrand factor (VWF) concentrate is recommended for individuals with von Willebrand disease (VWD) who have a history of frequent and severe bleeds, regardless of their age. The WIL-31 study demonstrated the efficacy of prophylaxis with a plasma-derived VWF/factor VIII (pdVWF/FVIII) concentrate containing VWF and FVIII in a 1:1 activity ratio (wilate ®) in adults and children with VWD of all types. Here we specifically report on the results in children and adolescents.
Aims: To investigate the efficacy and safety of regular prophylaxis with a pdVWF/FVIII concentrate in children and adolescents with VWD, compared with prior on-demand treatment.
Methods: WIL-31 (NCT04052698) was a prospective, non-controlled, international, multicenter phase 3 trial that enrolled male/female patients aged ≥6 years with VWD type 1 (VWF:RCo <30 IU/dL), type 2 (except 2N) or type 3. Prior to entering the WIL-31 study, all patients had received on-demand treatment with a pdVWF/FVIII concentrate during a 6-month, prospective, observational, run-in study (WIL-29); patients who experienced at least 6 BEs, excluding menstrual bleeds, with at least 2 of these BEs treated with a VWF-containing product, were eligible to enter WIL-31. Patients in WIL-31 received regular pdVWF/FVIII prophylaxis 2-3 times per week at a dose of 20-40 IU/kg for 12 months. In this post-hoc analysis patients were grouped by age: children (6-11 years old), adolescents (12-16 years old), and adults (≥17 years old). Mean total and spontaneous ABR were compared, and the number and site of breakthrough bleeds were described. Safety and tolerability were assessed throughout the study.
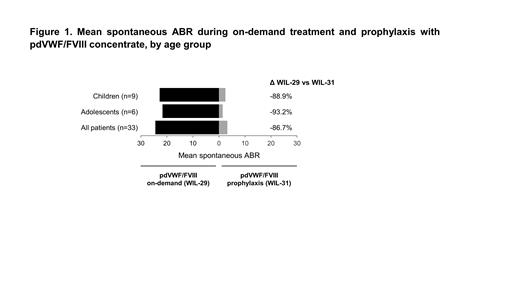
Results: The study population included 33 patients, with a median (range) age of 18 (7-61) years. Nine (27.3%) patients were children, 6 (18.2%) were adolescents, and 18 (54.5%) were adults. During 12 months of prophylaxis, 67% (6/9) of children and 83% (5/6) of adolescents experienced 34 (all minor) and 26 bleeds (20 minor; 6 major), respectively. Among children and adolescents, 82% (28/34) and 77% (20/26) of bleeds required treatment, with “excellent/good” efficacy achieved in 100% and 95% of treated bleeds, respectively. The mean total ABRs during on-demand vs prophylaxis were 32.5 vs 3.7 in children and 28.9 vs 4.3 in adolescents, representing 88.6% and 85.1% reductions during prophylaxis compared with on-demand treatment. Of these bleeds, the most common sites were nose (74%; 25/34) and oral cavity (9%; 3/34) for children, and nose (46%; 12/26), ankle (23%; 6/26) and elbow (19%; 5/26) joints for adolescents. During prophylaxis children experienced no joint bleeds; the mean knee, ankle, and elbow joint ABRs in adolescents were 0.2, 1.0, and 0.8, respectively. The mean spontaneous ABRs during on-demand treatment vs prophylaxis were 22.8 vs 2.5 (88.9% reduction) and 21.7 vs 1.5 (93.2% reduction) (Figure 1). Forty-four percent of children, and 33% of adolescents had zero spontaneous bleeding events during 12 months of prophylaxis. No serious adverse events related to study treatment and no thrombotic events were observed, and no safety concerns were raised by clinical laboratory, vital sign, and physical examination findings. There were no cases of parvovirus B19 seroconversion or inhibitor development judged to be related to study treatment.
Conclusion: Prophylaxis with pdVWF/FVIII was efficacious and well tolerated in children and adolescents with all types of VWD.
OffLabel Disclosure:
Sidonio:Takeda: Honoraria, Research Funding; Guardian Therapeutics: Honoraria; Bayer: Honoraria; Novo Nordisk: Honoraria; UniQure: Honoraria; Biomarin: Honoraria; Spark: Honoraria; Pfizer: Honoraria; Octapharma: Honoraria, Research Funding; Genentech: Honoraria, Research Funding. Dubey:Octapharma: Other: Clinical study investigator . Khayat:Octapharma: Honoraria; CSL Behring: Honoraria; LFB: Honoraria.
This study is investigating the use of a VWF / FVIII concentrate for the prevention of bleeds in patients with von Willebrand disease of any type