Introduction and methods: Mediastinal gray zone lymphoma (GZL) emerged as a rare and challenging entity due to its high rate of pathological reclassification and heterogeneous clinical management. The bio-GZL2020 study was designed to centralize histological samples and clinical data of patients (pts) diagnosed with GZL between 2006 and 2020 in 16 Italian hematological centers. We here report the preliminary clinical results of this retrospective analysis.
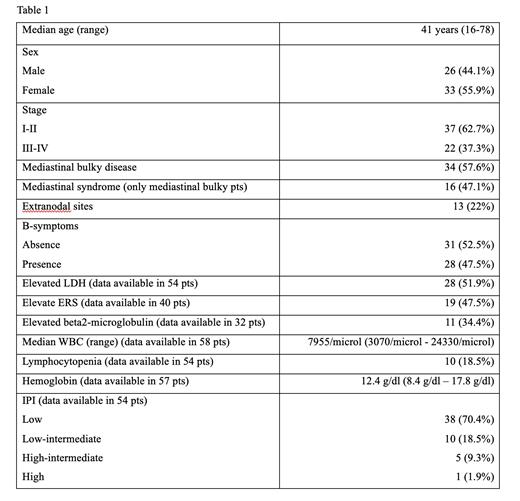
Results: Overall, we collected samples and clinical data of 68 pts with GZL. According to the 2022 WHO and ICC classifications we excluded nine pts for lacking mediastinal involvement, hence 59 pts were analyzed. Pts were mostly stage I-II (62.7%); 57.6% had a mediastinal bulky disease. The site of diagnostic biopsy was the mediastinum in 29 pts. Additional clinical characteristics at baseline are reported in Table 1. One patient died before the start of treatment and was excluded from survival analysis. First line chemotherapy (CHT) consisted in DA-EPOCH in 31 pts, CHOP-like regimens in 17 pts, ABVD in six pts, MACOP-B in three pts and DHAP in only one case. Eight pts did not receive rituximab with CHT (six ABVD pts, two CHOP-like pts). Dose escalation in R-DA-EPOCH was performed in 75% of pts, with 18 pts (64.3%) attaining level 3 or higher. Four advanced stage, high-risk pts received central nervous system prophylaxis. Twenty-two pts performed an interim PET-scan during first line therapy; data on Deauville score was available in 19 cases, with 9 pts obtaining a score 1-3. Restaging after first line CHT showed 37 pts in complete remission (CR; 63.8%), nine pts in partial remission (PR; 15.5%), five pts in stable disease (SD; 8.6%) and seven pts in progressive disease (PD; 12.1%). Thirty-one pts (53.4%) received a consolidative treatment (26 pts radiotherapy (RT) only, seven pts autologous transplant (ASCT) only, two pts both RT and ASCT). Restaging after first line consolidation showed 43 CR (74.1%), four PR (6.9%), two SD (5.2%) and eight PD (13.8%). Nineteen pts underwent a second line CHT, 16 of them with a platinum-based regimen; brentuximab vedotin, BeGEV and HyperCVAD were used in one patient each. Second line consolidation ASCT was performed in seven pts, while one received an allogeneic transplant. Of the 17 restaged pts after second line CHT seven were in CR, 10 were not responsive (four SD, six PD). Considering all salvage lines five pts received a checkpoint inhibitor, nine underwent brentuximab vedotin treatment. Overall survival (OS) was 86.9% at 5 years and 71.3% at 10 years; beta2-microglobulin (available only in 32 pts) and the response after first line therapy were associated to a statistically significant difference in OS in univariate analysis. Progression-free survival (PFS) was 61.9% at 5 and 10 years. The use of rituximab, the Deauville score 1-3 at interim PET during induction, and the response after first line therapy were associated to a statistically significant difference in PFS in univariate analysis. ABVD was associated to poorer results in terms of OS and PFS although no significant difference was observed. No statistically significant difference in term of PFS was observed between pts treated with intensified regimens (DA-EPOCH, MACOP-B, DHAP) and other treatments in first line. Excluding pts directly shifted to salvage therapy, consolidative RT after first line CHT showed no statistically significance in term of both OS and PFS.
Conclusions: To our knowledge our study represents the largest retrospective data collection on GZL among hematological Italian centers. This preliminary analysis did not show a clear advantage of intensive therapies such as DA-EPOCH or MACOPB, while the positive prognostic role of rituximab was confirmed in PFS. The prognostic role of interim PET-CT scan seems interesting and should be evaluated in subsequent trials. Further refinement of the reported results is expected in relation of an ongoing central pathological review by a panel of expert hematopathologists.
OffLabel Disclosure:
Nassi:Kiowa Kirin: Consultancy, Speakers Bureau; Janssen: Speakers Bureau; Incyte: Consultancy, Speakers Bureau; EUSApharma: Speakers Bureau; Eli Lilly: Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Roche: Consultancy. Botto:Takeda: Speakers Bureau. Paulli:Kyowa Kirin: Speakers Bureau. Fabbri:Incyte: Consultancy, Speakers Bureau; Takeda: Consultancy; Kyowa Kirin: Speakers Bureau. Balzarotti:Roche: Honoraria, Speakers Bureau; Novartis: Honoraria; Gilead: Honoraria, Speakers Bureau; Eli Lilly: Honoraria; Incyte: Honoraria, Speakers Bureau; Janssen: Honoraria, Research Funding; Takeda: Speakers Bureau; Kiowa Kirin: Speakers Bureau; Beigene: Research Funding; GenMab: Speakers Bureau. Annibali:Takeda: Speakers Bureau; Janssen: Speakers Bureau; Amgen: Speakers Bureau. Re:Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Italfarmaco: Membership on an entity's Board of Directors or advisory committees. Gini:Roche: Consultancy; Incyte: Consultancy; Takeda: Consultancy; Gentili: Consultancy. Sabattini:EUSApharma: Consultancy, Speakers Bureau; Recordati: Consultancy, Speakers Bureau; Kyowa Kirin: Speakers Bureau.
The study is a retrospective evalutation of patients with gray zone lymphoma. Some patients were treated with brentuximab vedotin or checkpoint inhibitors, which are currently off label.