Background: In patients (pts) with CML, the BCR::ABL1 T315I gatekeeper mutation confers treatment resistance to all approved ATP-competitive tyrosine kinase inhibitors (TKIs) except ponatinib and olverembatinib; thus pts harboring this mutation have limited treatment options.
Asciminib, a first-in-class TKI that works by specifically targeting the ABL myristoyl pocket (STAMP), has a distinct mechanism of action and no known overlapping resistance profile with ATP-competitive TKIs. In clinical trials, asciminib has demonstrated efficacy and acceptable safety in adult pts with Ph+ CML in chronic phase and the T315I mutation.
The asciminib MAP (NCT04360005) enables access to asciminib treatment for pts with CML who are resistant or intolerant to available treatments or for whom available treatments are contraindicated. This global chart review study aimed to better understand the effectiveness and safety of asciminib in pts with CML and the T315I mutation treated as part of the MAP.
Methods: This non-interventional chart review analyzed data from existing routine medical charts of pts enrolled in the MAP who received at least one dose of asciminib. Pts with CML aged ≥18 years old were included in the study if they had confirmed presence of the T315I mutation detected at any point prior to commencement of asciminib and had received their first dose of asciminib between 01 November 2018 and 30 April 2022. Data was abstracted from CML diagnosis date to the first dose of asciminib (index date) and up until 12 months after the start of asciminib.
Treatment effectiveness was assessed in terms of major molecular response (MMR) in pts in MMR at index date or not, at and by 3, 6, 9, and 12 months. Other variables included demographic and disease characteristics, treatment patterns, response to prior TKIs and safety. All analyses were descriptive.
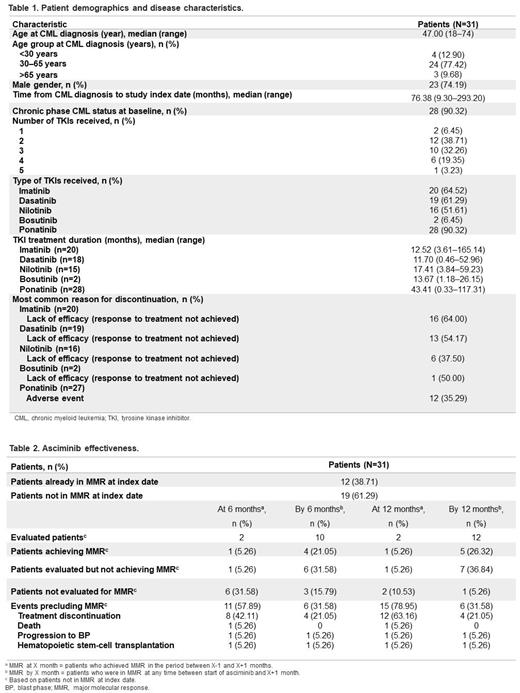
Results: 31 pts were included across 8 countries (Netherlands, UK, Italy, Hong Kong, Pakistan, Spain, Australia, and US). Median age at CML diagnosis was 47 (range 18‒74) years and most pts were in chronic phase ( Table 1). Pts were heavily pretreated, with the majority having received ≥2 prior TKIs. Nearly all pts (28/31; 90.3%) had received prior ponatinib treatment at any time before the switch to asciminib. Median prior TKI treatment duration was longest for ponatinib (43.4 months, range 0.3‒117.3) vs other TKIs.
The most common reason for discontinuation of all previous TKIs at any time before asciminib was lack of response to treatment, with the exception of ponatinib, where the most common reason was due to adverse events (AEs). Ponatinib was the last TKI prior to commencement of asciminib in 27/31 (87.1%) pts.
Overall, 22/31 pts (71.0%) experienced AEs that led to a switch to another TKI at any time prior to asciminib, whereas nearly half of pts discontinued the last TKI before starting asciminib due to intolerance (14 pts, 45.2%).
A total of 12/31 patients (38.7%) were already in MMR at index date ( Table 2), confirming that intolerance was an important reason for switching to asciminib. Of the pts not in MMR at the start of asciminib, 4/10 (40.0%) and 5/12 (41.7%) evaluated pts achieved MMR by 6 and 12 months, respectively.
Of the 12 pts in MMR at the start of asciminib, 6/12 (50.0%) maintained MMR by 6 months.
Overall, 10/31 pts (32.3%) discontinued asciminib before completion of the 12-month follow-up (death, 7 [4 serious AEs, SAEs; 2 CML; 1 unknown]; physician decision, 3). SAEs were reported in 9 pts (29.0%) that received asciminib; 8 SAEs led to hospitalization, 4 to death (1 cerebrovascular accident, 1 CML, 1 pneumonia, 1 fungal infection), 2 to asciminib discontinuation, and 2 were considered life-threatening. SAEs reported for 1 pt (hematotoxicity and procedural hemorrhage) were assessed as asciminib-related.
Conclusions: Asciminib demonstrated effectiveness in pts with CML and the T315I mutation in routine medical practice, including ponatinib pre-treated pts, a population with limited available treatment options. Effectiveness estimates were consistent with those observed in clinical trials. Although the number of pts was small, this global study reports on one of the largest cohorts of pts with CML and the T315I mutation.
OffLabel Disclosure:
Milojkovic:Pfizer: Honoraria; Novartis: Honoraria; Incyte: Honoraria. Castagnetti:Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria; Novartis: Consultancy, Honoraria, Research Funding. Gallipoli:Astellas: Honoraria. Westerweel:Novartis: Honoraria; Pfizer: Honoraria; Bristol Myers Squibb - Celgene: Honoraria; Incyte: Honoraria. Kell:Jazz: Other: Participated in advisory board meetings; Celgene: Other: Participated in advisory board meetings; Novartis: Other: Participated in advisory board meetings. Vachhani:Incyte, CTI BioPharma Corp, Blueprint Medicines: Speakers Bureau; Abbvie, Amgen, Blueprint Medicines, Cogent Biosciences, Incyte, CTI BioPharma Corp, Daiichi Sankyo, GlaxoSmith Kline, Karyopharm, Novartis, Pfizer, Genentech, Inc., Servier, Stemline, MorphoSys, LAVA therapeutics: Honoraria. Agarwal:Parexel International: Current Employment. Colasante:Parexel International: Current Employment. Meka:Parexel International Private Ltd: Current Employment. Smyth:Novartis: Current Employment. Ferreira:Novartis Pharmaceuticals Corporation: Consultancy, Current Employment. Janssen:Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Apps for Care and Science and non-profit foundation support ; Jazz: Other: Apps for Care and Science and non-profit foundation support ; Servier: Other: Apps for Care and Science and non-profit foundation support ; Sanofi Gemzyme: Other: Apps for Care and Science and non-profit foundation support ; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Apps for Care and Science and non-profit foundation support ; Olympus: Other: Apps for Care and Science and non-profit foundation support ; Janssen: Other: Apps for Care and Science and non-profit foundation support ; Daiichi-Sankyo: Other: Apps for Care and Science and non-profit foundation support ; Astellas: Other: Apps for Care and Science and non-profit foundation support ; Amgen: Other: Apps for Care and Science and non-profit foundation support ; BMS: Other: Apps for Care and Science and non-profit foundation support , Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Asciminib has been approved in several countries for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukemia (Ph+ CML) in chronic phase (CP), previously treated with 2 or more tyrosine kinase inhibitors (TKIs). In October 2021, asciminib was also approved for the treatment of patients with Ph+ CML in CP with the T315I mutation in the US.