Background Myeloidblast phase of chronic myeloid leukemia (CML-MBP) has a terrible prognosis with a haemopoietic cell transplant the only likely cure. Probability of transplant success is markedly increased if someone can be returned to chronic phase.
Purposes Study safety and efficacy of olverembatinib or ponatinib and azacitidine (AZA) in this setting.
MethodsCML-MBP was diagnosed according to the WHO 2016 criteria. Ponatinib, 45 mg/d or olverembatinib, 30 mg every other d were combined with azacitidine, 75 mg/mE+2/d for 7 d in 28 d cycles. Subjects achieving 2 nd chronic phase received an allotransplant whereas others continued the therapy specified. 2 nd chronic phase was defined as < 10% blood and bone marrow blasts and no extra-medullary leukaemia for ≥ 4 w. Event-free survival (EFS) was defined as interval from therapy start to no response, progression to accelerated or blast phase or CML-related death. CML-related survival was defined as interval from therapy start to death from blast phase and survival, this interval to death from any cause. Haematologic, cytogenetic and molecular responses were monitored. Targeted DNA sequencing and whole RNA sequencing were done before therapy start. “MOVICS” package in R software was used for multi-omics integrated analyses and molecular sub-type classification. Cox regression models were used to identify co-variates associated with outcomes. The study was registered in Chinese Clinical Trial Registry (ChiCTR2200055887).
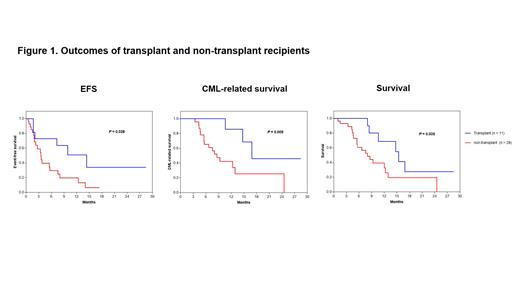
Results 39 consecutive subjects were registered from October, 2020 to July, 2023. 21 were male. Median age was 50 years (Interquartile range [IQR], 33-54 years). 23 received olverembatinib; 16, ponatinib. Median follow-up was 10 months (mo) (IQR, 3-19 mo). 30 (77%) subjects achieved a 2 nd chronic phase after 1 cycle, 11 of whom received a transplant, 8 progressed to blast phase again; and 9 (23%) others had no response. 25 subjects died of progression (N = 19), transplant-related mortality (N = 3), COVID-19 (N = 2) or heart failure (N = 1). Median EFS, CML-related survival and survival were 4 (95% Confidence Interval [CI], 1-6) months, 12 (8-17) months and 11 (6-15) months. Subjects receiving a transplant had better EFS ( p= 0.03), CML-related survival ( p= 0.009) and survival ( p= 0.04) compared with non-transplant subjects ( Figure 1). Most treatment-related severe adverse events were tolerable and manageable including 25 subjects (65%) developing ≥ 4 grade neutropenia with or without thrombocytopenia except only 1 subject developing early death due to heart failure. In addition, 2 subjects experienced COVID-19 and died subsequently.
In adjusted Cox regression analyses of genomic (N = 37) and transcriptomic (N = 34) data KRASmutation was significantly-associated with worse EFS ( p= 0.05), CML-related survival ( p< 0.001) and survival ( p= 0.009). TP53mutation was associated with worse CML-related survival ( p= 0.02) and survival ( p= 0.003). PFKFB3::LINC02649 fusion was associated with better EFS ( p= 0.001), CML-related survival ( p= 0.007) and survival ( p= 0.005). Based on these data 34 subjects were divided into 2 clusters. Subjects in cluster 2 (N = 14) had worse EFS ( p= 0.001), CML-related survival ( p= 0.008) and survival ( p= 0.005) compared with those in cluster 1 (N = 20). In differential gene expression analyses 1,564 genes were up-regulated and 1,000 down-regulated in cluster 2 compared with cluster 1. In KEGG analyses up-regulated genes were enriched in signaling pathways including cytokine-cytokine receptor interaction, cell adhesion molecules, natural-killer (NK) cell mediated cytotoxicity, primary immune deficiency and Toll-like receptor. Down-regulated genes were enriched in cell-cycle and DNA replication pathways.
Conclusion s Olverembatinib or ponatinib combined with azacitidine is a safe and effective therapy of CML-MBP allowing many subjects to receive a transplant in 2 nd chronic phase. KRAS and TP53 mutations and PFKFB3::LINC02649 fusions had predictive impact on outcomes. Determining whether adding azacitidine to a 3 rd-generation TKI requires a randomized controlled trial.
Disclosures
No relevant conflicts of interest to declare.