Background
Impaired immune cell reconstitution after allogeneic hematopoietic stem cell transplantation (allo-HSCT) poses risks for both disease recurrence and treatment-related complications, such as cytomegalovirus (CMV) reactivation. CMV strongly shapes the immune system, but the development of anti-CMV T cells and CMV-associated adaptive NK cells in vivo is still largely uncharacterized. Understanding these dynamics post-allo-HSCT may have clinical implications, since NK cells mediate a graft-versus-leukemia effect, particularly in myeloid malignancies, and adaptive NK cells have recently been associated with favorable immunotherapy responses in both hematological and solid cancers.
Here, with longitudinal sample collection pre and post-allo-HSCT, we aimed to determine how patient and donor-derived NK and T cells synergistically contribute to immune system regeneration, how clonotypes develop and persist, and whether donor/recipient CMV serostatus and CMV reactivation post-allo-HSCT influence NK and T cell phenotypes.
Methods
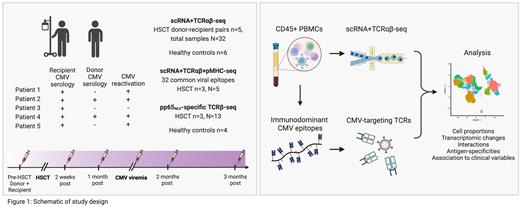
We sequenced 32 longitudinal CD45+ sorted peripheral blood samples with single-cell RNA and T cell receptor sequencing (scRNA+TCRαβ-seq) from 5 allo-HSCT donor-recipient pairs (pre-transplant sample from donor and recipient and 3-4 follow-up samples per patient over 3 months) and 6 healthy controls (Figure). All recipients were CMV seropositive, while 2 and 3 donors were seropositive and negative, respectively. CMV reactivation occurred in 4 patients during follow-up, on average 32 days post-allo-HSCT.
We screened 32 common viral epitopes from post-transplant samples (n=3, 5 samples) with scRNA+TCRαβ+pMHC-seq (Immudex dCODE Dextramers). We then profiled T cells specific to CMV pp65 NLV-multimer with TCRβ-seq (allo-HSCT donor-recipient pairs n=3, 13 samples, healthy n=4), and traced CMV-recognizing TCRs in the scRNA+TCRαβ-seq data.
Results
We analyzed a total of 197,887 immune cells, including 37,123 NK cells and 59,852 CD8+ T cells. After allo-HSCT, an early increase in classical CD14+ monocytes was observed, followed by expansion of NK and T cell populations. At the time of CMV viremia, NK cell and CD8+ T effector memory/terminally differentiated effector memory (CD8+ T EM/EMRA) populations increased regardless of donor CMV serology.
CD56 bright, CD56 dim and adaptive NK cells were present in all patients post-transplant, with patient-specific heterogeneity in phenotype and temporal dynamics. The proportion of adaptive NK cells out of CD45+ cells was higher post-viremia in 2 patients with CMV+ donors (18.9% and 7.4%), compared to 2 patients with CMV- donors (3.1% and 1.7%). Surprisingly, the expansion of adaptive NK cells (3.8%) was also noted in a patient with a CMV- donor without CMV reactivation post-allo-HSCT. After viremia, adaptive NK cells upregulated IFNG production, KLRC2/NKG2C and KLRC3/NKG2E and downregulated KLRC1/NKG2A.
CD8+ T cell clonality markedly increased after CMV viremia in all patients with CMV reactivation, as measured by proportion of large and hyperexpanded clones in the scTCRαβ-seq data. Hyperexpanded clones consisted mostly of CD8+ T EM/EMRA phenotypes and were present only in samples collected during or after viremia.
With scRNA+TCRαβ+pMHC-seq, pp65 NLV was discovered to be the immunodominant CMV epitope. Characterizing pp65 NLV-specific TCRs in the scRNA+TCRαβ data, CMV-specific CD8+ T cells belonged to highly clonal T EM/EMRA clusters. Further, a subset enriched to a T EMRA phenotype expressing NK cell receptors, including KIR3DL2, KIR2DL3, KLRC2/NKG2C KLRC3/NKG2E, and FCGR3A/CD16, and the exhaustion markers TIGIT, TOX, and CD160 at a higher level than other CD8+ T cells. The donor-derived CMV-specific TCR repertoire evolved in recipients after viremia. For example, in patient 2 we noted an 18.5-fold expansion of a donor-derived CMV-specific clone during viremia that was not originally the most dominant anti-CMV T cell clone in the donor.
Conclusions
We delineated NK cell and antigen-specific T cell dynamics in the unique setting of allo-HSCT and CMV reactivation, highlighting the manyfold expansion of donor-derived T cell clones and the expansion and evolution in the transcriptional profile of adaptive NK cells during CMV viremia. Our study sheds light on the immunobiology of these two cell types with implications in transplant outcome and translational relevance as cancer immunotherapy.
Disclosures
Myllymäki:Gilead Sciences: Research Funding; Sanofi: Honoraria; Celgene: Honoraria. Mustjoki:Novartis: Honoraria, Research Funding; Pfizer: Research Funding; Dren Bio: Honoraria; BMS: Honoraria, Research Funding.