Introduction: With the wider use of post-transplant cyclophosphamide (PTCy) graft-versus-host disease (GVHD) prophylaxis, there is interest in replacing tacrolimus (TAC) with sirolimus (SIR, mTOR inhibitor) to avoid calcineurin inhibitor toxicity, maintain excellent GVHD control, and potentially allow for a greater graft-versus-tumor effect. mTOR and Aurora kinase A (AURKA) mediate CD28 costimulation in alloreactive T cells. SIR can only partially block CD28 costimulation. Adding selective AURKA inhibition to SIR following PTCy fully suppresses CD28 signal transduction, with synergistic GVHD prevention in mice (see ASH 2023 abstract ID 173376). AURKA inhibitors also have direct anti-leukemia activity. Here, we present the phase 1 dose escalation portion of a first-in-human clinical trial of PTCy plus SIR and VIC-1911 (VIC), a selective oral AURKA inhibitor, for GVHD and relapse prophylaxis after myeloablative allogeneic hematopoietic cell transplantation (alloHCT).
Methods: This single-arm phase I dose escalation trial (NCT05120570) is designed to evaluate the pharmacodynamic potency of AURKA pathway suppression and the safety of PTCy/SIR/VIC. The primary endpoint of phase I is to identify the lowest biologically active dose of VIC, defined as <54% pH3ser10+ CD4+ T cells at day +21 (the lower confidence interval of the normal, pre-transplant frequency of pH3ser10+ CD4+ T cells - a marker of AURKA activity), that is safe with PTCy/SIR. Patients aged 18-60 years receive myeloablative conditioning of TBI alone at a dose of 1320 cGy. PTCy is given as 50 mg/kg on days +3 and +4, SIR on day +5 with levels targeting 8-12 ng/ml and tapered after day +100, and VIC given on days +5 to +45 at doses of 25, 50, or 75 mg BID. Patients undergoing myeloablative TBI-based alloHCT with PTCy/SIR plus mycophenolate mofetil (MMF), without VIC on a separate protocol, served as a control.
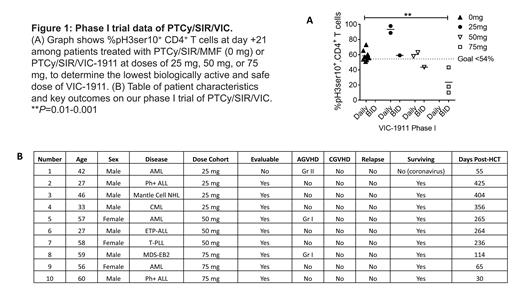
Results: Proof-of-concept experiments with PTCy/SIR/alisertib (AURKA inhibitor) demonstrate synergistic protection against xenogeneic GVHD and tumor (Raji cells) in mice, compared to PTCy/TAC (median survival: 45 days v undefined, 9 mice/group, 2 experiments, P<0.0001). Unlike alisertib, VIC-1911 is not myelosuppressive, providing rationale for this trial. In vitro studies show killing of multiple leukemia cells lines at physiologically achievable VIC-1911 concentrations. VIC 75 mg BID is the lowest biologically active dose suppressing AURKA activity below the primary endpoint target of <54%, with reduced mean frequency of day +21 pH3ser10+ CD4+ T cells at 23.7% compared to 58.5% with PTCy/SIR/MMF (Figure 1A, P=0.0091). PTCy/SIR/VIC also suppresses mTOR activity in donor T cells (3.9% v 16.6% pS6+ CD4+ T cells, P=NS; pS6 frequency in healthy donor T cells is ~30-40%), concurrently ablating CD28 signal transduction. Data trends show greater Tregs at day +21 with PTCy/SIR/VIC, compared to PTCy/SIR/MMF controls (40.2% v 10.5%, P=NS). Patient characteristics are detailed in Figure 1B. Neutrophil engraftment occurred in 9/9 evaluable patients at a median of 18 days. Platelet engraftment occurred in 8/9 evaluable patients at a median of 21 days; one patient is awaiting platelet engraftment. No dose-limiting toxicities have been observed during dose escalation. Patient 1 died of non-COVID coronavirus during initial hospitalization; he received high-dose methylprednisolone for ARDS beginning on day +17, which made him unevaluable for the primary pharmacodynamic endpoint.
Conclusions: A VIC-1911 dose of 75 mg BID from day +5 to day +45 effectively suppresses AURKA activity as determined by a low frequency of pH3ser10+ CD4+ T cells, ablating CD28 T cell costimulation when combined with sirolimus, resulting in no dose-limiting toxicities. VIC 75 mg BID will be studied further in an expanded phase I cohort to obtain estimates of efficacy in preventing both GVHD and relapse in PTCy-based myeloablative alloHCT.
OffLabel Disclosure:
Holtan:Sanofi: Research Funding; Incyte: Research Funding; Vitrac: Research Funding; Ossium: Consultancy; CSL Behring: Other: Endpoint Adjudication Committee. Maakaron:Atara: Research Funding; CLBR: Research Funding; Precision Biosciences: Research Funding; CRISPR: Research Funding; FATE Therapeutics: Research Funding; Gilead: Research Funding. Bachanova:ADC: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Other: DSMB; BMS: Research Funding; Citius: Research Funding; Incyte: Research Funding; Gamida Cell: Research Funding. Myers:Strategia Therapeutics: Current Employment; Vitrac Therapeutics: Current Employment; JSI Ventures: Current equity holder in private company. Paradiso:Strategia Therapeutics: Current Employment; Vitrac Therapeutics: Current Employment; JSI Ventures: Current equity holder in private company. DeFor:National Marrow Donor Program: Current Employment. Betts:Incyte: Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Research Funding; CRISPR: Patents & Royalties; Vitrac Therapeutics: Research Funding.
VIC-1911 is being tested for GVHD and relapse prevention.