Intestinal colonization with antibiotic-resistant bacteria has been associated with increased risk of systemic infections after allogeneic hematopoietic cell transplantation (HCT) and anti-leukemia induction chemotherapy. Fecal microbiota transplantation (FMT) has been used with success in gut decolonization from select antibiotic-resistant pathogens in small series. Large-scale data using expanded panels of antibiotic-resistance genes (ARGs) and long-term effects of FMT are unavailable. We performed a correlative analysis of samples from a prospective trial to address this knowledge gap. We conducted a randomized double-blind trial of oral, encapsulated, third-party fecal microbiota transplantation (FMT) versus placebo at the time of neutrophil recovery in two independent cohorts of patients with AML undergoing induction chemotherapy or allogeneic transplant recipients, with the primary objective of reducing infections (ClinicalTrials.gov identifier: NCT03678493; J. Clin. Oncol 2023 May 26). 100 patients (HCT: 74, AML: 26) were randomized between FMT vs. placebo, each given as 5 oral capsules taken at once a the time of neutrophil engraftment. FMT was safe and ameliorated intestinal dysbiosis, but the trial did not meet its primary endpoint. Longitudinal pre- and post-treatment stool samples were collected at the following timepoints: baseline (before starting conditioning; T0), pre-dose 1 (T1), day 10 post-dose 1 (T2), day 28 post-dose 1 (T3), and at the end of study follow up (9 months; T4).
All stool samples underwent 16S rRNA gene sequencing for the pre-planned microbiota endpoints of the trial. 226 samples with remaining DNA were used for microfluidic qPCR to simultaneously detect and quantify 47 ARGs including those for beta-lactams, quinolones, vancomycin, and carbapenem resistance. All samples were run in duplicate and their average gene content for each ARG (gene copies/microL DNA) was calculated using standard curves of the respective genes. Both qualitative (presence/absence; Fisher's exact test) and quantitative (Wilcoxon's test) analyses were performed, with a 0.05 threshold for P values defining statistical significance. The small number of T3 samples (n = 27) did not allow a meaningful comparison between the two arms; these samples were not included in analysis.
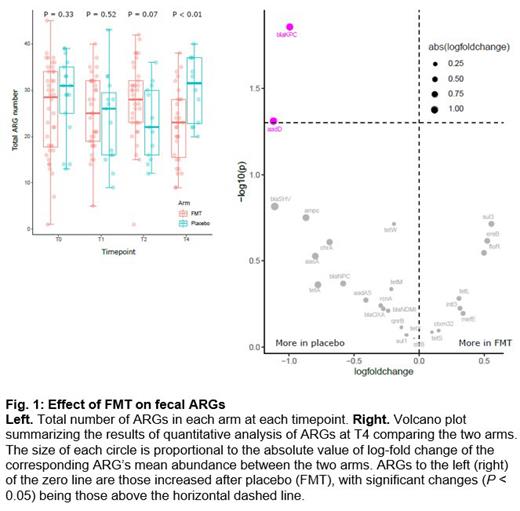
As expected from the randomized design, there was no difference between the FMT and placebo arms in the number of ARGs at T0 or T1 (median [range]; T0: 29 [1-45] vs. 31 [13-39], P = 0.33; T1: 25 [5-40] vs. 26 [9-43], P = 0.52). There was a trend for more ARGs at T2 in the FMT arm (28 [1-42] vs. 22 [12-36], P = 0.07). At T4, there were fewer ARGs in the FMT arm compared to placebo (23 [9-38] vs. 32 [20-40], P < 0.01; Fig. 1 left panel). In quantitative analysis comparing the two arms at each post-treatment timepoint, sul1 (sulfonamide), floR (chloramphenicol), ctxm32 (beta-lactam), qnrB (quinolone), tetS (tetracycline), and aadA5 (aminoglycoside) were more abundant in the FMT arm at T2. At T4, blaKPC (carbapenem) and aadD (aminoglycoside) were more abundant in the placebo arm ( Fig. 1 right panel). Given our findings at T2, we evaluated whether ARGs in post-treatment samples were also present in pre-treatment samples from the same patient. In the case of FMT, de novo appearance of an ARG post-treatment could indicate ARG transmission via FMT. Although donor stool was screened for a panel of clinically relevant ARGs and pathogens, some ARGs may be found in healthy individuals and could have been present in the FMT product. Using a per-ARG Fisher's exact test, no ARG appeared de novo post-FMT more frequently than after placebo, arguing against ARG transmission via FMT.
Findings from this largest randomized trial of FMT in AML patients and HCT recipients to date indicate long-term efficacy of FMT in antibiotic-resistant gut decolonization. A temporary early phase of reduced colonization resistance after FMT, possibly due to drastic, rapidly changing rewiring of microbiota networks, seems to occur, followed by formation of more stable communities resistant to ARG colonization. Clinical translation of these findings requires further research.
Disclosures
Rashidi:Seres Therapeutics, Ltd.: Consultancy. Holtan:Sanofi: Research Funding; Ossium: Consultancy; Incyte: Research Funding; Vitrac: Research Funding; CSL Behring: Other: Endpoint Adjudication Committee.