Introduction
Cold agglutinin disease (CAD) is an autoimmune hemolytic anemia that is rare and poorly understood. This means that clinicians can find understanding the immunopathogenesis of CAD and individualizing therapy challenging, and may struggle to maintain their knowledge of current and emerging therapies, so patients may not receive optimal care. Therefore, we designed an easily accessible, online, continuing medical education activity for hematologists and hematologist-oncologists, using quality improvement principles together with other change approaches, to improve the care clinicians provide to patients with CAD.
Methods
The online activity included 3 short videos presented by faculty who shared their expertise in managing patients with CAD. We set learning objectives based on key topics we identified as being challenging for clinicians. This allowed us to measure the impact of the education on knowledge, competence (based on Moore's outcomes framework Levels 3 and 4), intention to change practice and clinical confidence. We also measured participation and satisfaction (Moore's Levels 1 and 2), and changes by region (EU5, Latin America and USA) and clinician experience. Questionnaires were fielded by an independent third party (Nuaxia Limited [Richmond, UK]) at baseline (n=100) and within 3 months (n=100) and 6 months (n=50) after participation in the activity to assess knowledge, competence, satisfaction, intention to change practice and confidence. Additionally, these assessments allowed us to identify the need for further educational activities and the topics they should cover. Participation was measured using Google Analytics. Questionnaire responses were analyzed by touchIME statisticians and data from 3 and 6 months were pooled for combined analysis. The margin of error was calculated as ~3% based on a standard confidence interval of 95%.
Results
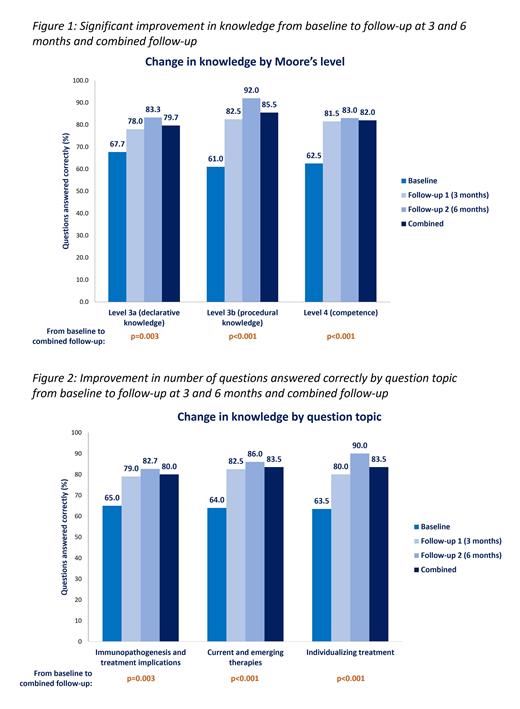
At 6 months, the combined analysis showed an increase from baseline in declarative and procedural knowledge, and competence of 12%, 25% and 20%, respectively (Figure 1). These improvements did not show any significant variation by geography (p=0.432) or by years in practice (p=0.923). Analysis by question topic showed that the significant improvements were achieved consistently (p=0.003) for immunopathogenesis, p<0.001 for current and emerging therapies, and p<0.001 for individualizing treatments; Figure 2). The total number of questions answered correctly also increased significantly (p<0.001) from baseline (64.3%; SD 1.808) at the 6-month combined follow-up (82.0%; SD 1.808). The combined analysis showed clinician confidence in managing CAD improved from 44%, being moderately or extremely confident at baseline, to 72% at 6 months; a median of 57% of learners said they would make a change in their clinical practice. At 6 months post-launch, 6,754 participants had engaged with the education and the overall satisfaction was 88%.
Conclusion
This educational activity led to significant improvements in the knowledge and competence of hematologists and hematologist-oncologists in the understanding and management of CAD. Further, the majority of participants stated they were more confident and would change their clinical practice, which may improve the quality and effectiveness of patient care. The short, accessible format of this activity meant clinicians could participate whenever convenient and the use of expert-led videos made it engaging and applicable, which may have contributed to the success of the activity. Feedback from learners and our analysis indicate that further quality improvement initiatives on this topic would be beneficial and should cover integrating novel therapies into the clinic, as well as improving diagnostic accuracy in the non-speciality clinic.
Disclosures
Jilma:Sanofi: Consultancy, Honoraria, Speakers Bureau; Guardian Therapeutics: Consultancy. Gertz:Ionis/Akcea: Honoraria; Celgene: Honoraria; Ashfield: Honoraria, Research Funding; Aptitude: Honoraria; Prothena: Honoraria; Sanofi: Honoraria; Sorrento: Honoraria; Johnson & Johnson: Honoraria; Juno: Research Funding; Janssen: Honoraria; AbbVie: Honoraria. D'Sa:Kite: Consultancy; Sanofi: Consultancy, Honoraria; Janssen: Honoraria, Speakers Bureau; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau. Boothman:Pulsar Healthcare / Integrated medhealth communication: Ended employment in the past 24 months. Kazi:AS&K Communications (Remedica): Ended employment in the past 24 months.