Introduction
Metformin is a commonly used medication for the treatment of Type 2 Diabetes Mellitus (T2DM). It has also been hypothesized to reduce the absorption of vitamin B12 in the GI tract. Development of B12 deficiency due to metformin-associated reduced absorption would take years to develop, because vitamin B12 has sufficient stores for 3-10 years. As such, the present systematic review and meta-analysis aims to evaluate the effect of metformin on anemia and vitamin B12 deficiency, with outcomes stratified by duration and dosage of metformin exposure.
Methods
Electronic searches were conducted on MEDLINE, EMBASE, Web of Science, and the Cochrane Central Register of Controlled Trials from inception to June 2023, supplemented with manual citation search. Two reviewers independently and in duplicate assessed titles, abstracts, and full-text articles, with discrepancies resolved by a third member. Studies considered for inclusion were randomized controlled trials (RCTs) and non-randomized studies (NRS) in any language, evaluating anemia risk in T2DM patients using metformin compared to standard-of-care non-metformin treatments for T2DM .
The primary outcome was frequency of anemia. Secondary outcomes included frequency of vitamin B12 deficiency, changes in serum hemoglobin and vitamin B12 levels, folate deficiency, changes in serum folic acid, and homocysteine levels. Results were stratified based on the Metformin Use Index (MUI), which categorizes daily metformin dose in mg x duration in years/1000 into three subgroups: MUI ≤ 5, MUI > 5, and MUI Unspecified (when the primary study did not report or stratify based on metformin dosage and duration). Mantel-Haenszel random effects model was used to synthesize binary and continuous endpoints, providing odds ratios (OR) and mean differences (MD) with 95% confidence intervals (CI).
Results
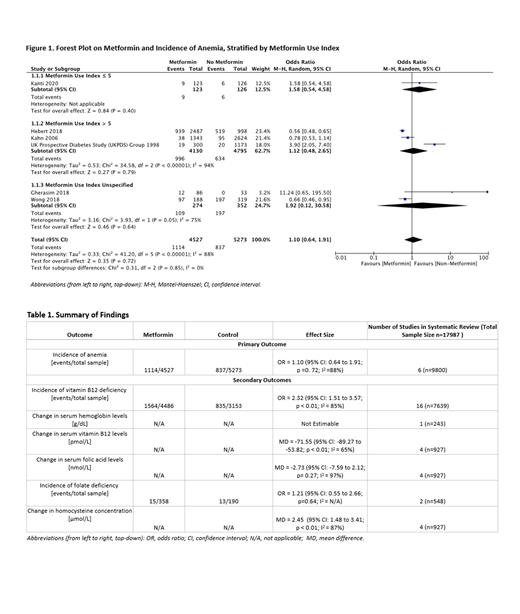
7443 studies were retrieved from electronic databases and 26 studies comprising 6 RCTs, and 20 NRS were included. In total, 17343 patients were included in this systematic review. Overall, the risk of anemia did not significantly differ between the metformin and control group (OR 1.10 [95%CI 0.64, 1.91]). Subgroup analysis also revealed no significant differences between metformin and control in the MUI ≤ 5 group (OR 1.58 [95%CI 0.54, 4.58]) and the MUI > 5 group (OR 1.12 [95%CI 0.48, 2.65]).
Metformin was associated with an increased risk of vitamin B12 deficiency compared to control (OR 2.32 [95%CI 1.51, 3.57]). Subgroup analysis demonstrated that the difference was only significant in the MUI > 5 (OR 3.57 [95%CI 2.98, 4.27]) and the MUI Unspecified groups (OR 2.24 [95%CI 1.48, 3.38]), but not in the MUI ≤ 5 group (OR 1.01 [95%CI 0.83, 1.23]). The subgroup differences were significant (p<0.01). Metformin was associated with a decrease in serum vitamin B12 level (MD -71.55 pmol/L [95%CI -89.27, -53.82]), and change in serum hemoglobin could not be pooled given only one primary study. Serum homocysteine was higher in the metformin group (MD 2.45 µmol/L [95%CI 1.48, 3.41]), and there were no significant differences between metformin and control in both incidence of folate deficiency and change in serum folic acid.
Conclusion
Metformin use does not appear to increase the risk of anemia compared to placebo or other standard-of-care treatments in T2DM. Vitamin B12 deficiency is associated with metformin use, however likely only in those with longer/greater-dose (metformin use index > 5). Monitoring and supplementation of vitamin B12 level are likely beneficial in this population. Our study is limited by heterogenous criteria for anemia and vitamin B12 deficiency across jurisdictions. Further studies on the safety profile of metformin are recommended to include anemia as an outcome and result-stratification based on metformin dose and duration.
Disclosures
Crowther:CSL Behring: Honoraria; Treasurer, American Society of Hematology: Membership on an entity's Board of Directors or advisory committees; Bayer: Honoraria; Eversana: Consultancy; Syneos Health: Consultancy; Hemostasis Reference Laboratory: Consultancy; Precision Biologics: Consultancy; Astra-Zeneca: Consultancy; Pfizer: Honoraria.