Background: Pyruvate kinase (PK) deficiency is a rare, hereditary disorder that leads to chronic hemolytic anemia and significant complications, including iron overload. Mitapivat is a first-in-class, oral, allosteric activator of PK, approved in the United States by the Food and Drug Administration for the treatment of hemolytic anemia in adults with PK deficiency, and in the European Union by the European Medicines Agency and in Great Britain by the Medicines and Healthcare products for the treatment of PK deficiency in adults. In the ACTIVATE trial (NCT03548220), mitapivat demonstrated improvements in hemoglobin (Hb) in patients (pts) who were not regularly transfused. 40% (16/40) of pts receiving mitapivat met the primary endpoint of Hb response (≥1.5 g/dL increase from baseline [BL] sustained at ≥2 scheduled assessments at Weeks [Wks] 16, 20, and 24 in the fixed-dose period) compared with 0 for placebo (PBO). In a post-hoc analysis, 3 pts who did not meet the protocol-defined endpoint in ACTIVATE later achieved Hb improvements ≥1.5 g/dL after the start of the fixed-dose period and into the long-term extension (LTE). Factors contributing to delayed improvements in Hb with mitapivat have not yet been explored, but in the setting of chronic hemolysis, iron overload may interfere with erythropoiesis and limit Hb response.
Methods: In the global, phase 3, randomized, PBO-controlled ACTIVATE trial, 80 pts were randomized 1:1 to receive mitapivat or PBO for a 12-wk dose-optimization period (5/20/50 mg twice daily), followed by a 12-wk fixed-dose period. Pts who completed the ACTIVATE trial were eligible to continue into the LTE where all pts received mitapivat (pts randomized to PBO in ACTIVATE that received mitapivat in LTE completed a 12-wk dose-optimization period [5/20/50 mg twice daily] and then continued on optimized mitapivat dose). This analysis examined changes in markers of hemolysis and liver iron concentration (LIC) in pts who did not meet the protocol-defined endpoint (≥1.5 g/dL increase from BL sustained at ≥2 scheduled assessments at Wks 16, 20, and 24 in fixed-dose period), but achieved a delayed Hb response, defined as ≥1.5 g/dL increase from BL in Hb at least twice, with the 2nd improvement not occurring until the start of the LTE. Data for Hb and LIC are reported up until the last time point for which data were available for all pts.
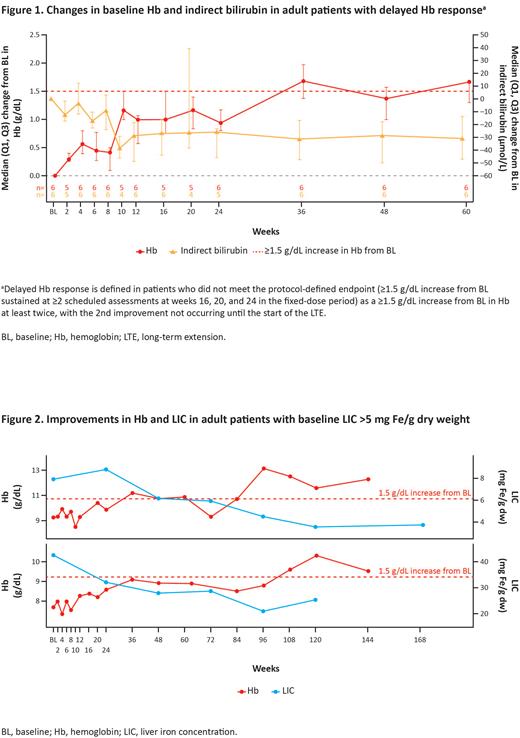
Results: A total of 6 pts (3 originally randomized to mitapivat and 3 originally randomized to PBO; all received mitapivat in the LTE) met the criteria for delayed Hb response which occurred between Wks 36 and 120 after the start of mitapivat treatment. Median (Q1,Q3) improvements from BL in Hb were 1.7 (1.3, 1.7) g/dL at Wk 60 (Figure 1). Five of 6 pts showed early improvements in hemolysis. Wk 12 median (Q1,Q3) change from BL in indirect bilirubin was -28.3 (-48.6, -18.5) µmol/L (Figure 1) and in reticulocyte percentage was -11.5 (-19.6, -3.7) %. Decreases in LIC occurred in 5 of 6 pts with median (Q1,Q3) change from BL in LIC of -1.3 (-2.0, -0.2) mg Fe/g dry weight at Wk 72 (measured every 24 wks). Two of 6 pts had BL LIC >5 mg Fe/g dry weight. Both pts (one on chelation) experienced improvements in LIC which corresponded with increases in Hb (Figure 2).
Conclusion: Adult pts with PK deficiency who do not show early Hb improvements (≥1.5 g/dL) with mitapivat may still experience a delayed Hb response, generally preceded by improvements in hemolysis. In addition, pts with BL iron overload may experience decreases in LIC that correspond to increases in Hb. Improvements in hemolysis and LIC during mitapivat treatment may allow for an eventual Hb response when early Hb improvements ≥1.5 g/dL are not observed.
Disclosures
Van Beers:Novartis AG: Research Funding; Pfizer, Inc.: Research Funding; Agios Pharmaceuticals, Inc. Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; RR Mechatronics: Research Funding. McGee:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Xu:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Dibacco:Agios Pharmaceuticals, Inc.: Current Employment, Current equity holder in publicly-traded company. Kuo:Pfizer: Consultancy; Bioverativ/Sanofi/Sangamo: Membership on an entity's Board of Directors or advisory committees; Novo/Nordisk: Consultancy, Honoraria; Vertex Pharmaceuticals: Consultancy; Forma Therapeutics: Consultancy; Bristol Myers Squibb: Consultancy, Honoraria; Alexion Pharmaceuticals: Consultancy; Agios Pharmaceuticals: Consultancy, Research Funding. Al-Samkari:Moderna: Consultancy; Pharmacosmos: Consultancy; argenx: Consultancy; Amgen: Research Funding; Novartis: Consultancy; Sobi: Consultancy, Research Funding; Agios: Consultancy, Research Funding.