Introduction: The management of light-chain (LC) monoclonal gammopathy of undetermined significance (MGUS) presents inherent challenges as the key diagnostic factor - abnormal free-light chain (FLC) levels - only indirectly implies the presence of an underlying clonal cell population. Moreover, the lack of M protein, used in current risk-stratification models, necessitates a distinct approach for risk assessment in LC-MGUS. Using next-generation flow cytometry (NGF), we assessed clonal plasma cell presence in bone marrow (BM) samples from individuals with LC monoclonal gammopathies. This approach enabled us to evaluate the predictive value of FLC ratio for an underlying clonal plasma cell population in LC-MGUS and its prognostic implications.
Aim: To evaluate the association between FLC ratio and the presence of clonal plasma cells in the BM of individuals with LC monoclonal gammopathies as well as the risk of progression.
Methods: The study is a part of the Iceland Screens, Treats, or Prevents Multiple Myeloma (iStopMM) study, a population-based screening study for multiple myeloma (MM) precursors and randomized trial of follow-up strategies. We included screened individuals with an abnormal FLC ratio (<0.26 or >1.65) and elevated involved LC (kappa ≥19.4 mg/L or lambda ≥26.3 mg/L) (Freelite, The Binding Site), with no detected M protein. Those with previously known MM or related disorders were excluded. NGF was used to determine clonal plasma cell presence in BM samples. Logistic regression was used to assess the relationship between FLC ratio (used as involved/uninvolved ratio throughout the study) and clonal plasma cell presence. Receiver operating characteristic (ROC) curve analysis was used to assess the predictive ability of FLC ratio for clonal plasma cell presence in LC-MGUS and to determine an optimal cutoff value. Kaplan-Meier analysis was used to assess time to progression to more advanced plasma cell disorder for LC-MGUS, stratified by the FLC ratio cutoff. A linear mixed-effects model was used to evaluate the change in FLC ratio (expressed as fold change relative to the screening sample) over time for those with FLC ratio below the cutoff at screening.
Results: After screening 75,422 individuals, 1,965 with LC disease were included, with median age of 71 years (range of 42-100 years), and a median FLC ratio of 1.85. Most (95.4%) had involved FLC of type kappa. Clonal plasma cells were identified by NGF in 8/21 (38.1%) LC-MGUS, and all LC-smoldering MM (SMM) (n=21) and LC-MM (n=7) cases.
A significant prediction of clonal plasma cell presence was found for FLC ratio (OR=6.47; p=0.012; n=49), supported by ROC curve analysis for LC-MGUS (AUC=0.94; n=21), identifying FLC ratio of 3.15 as optimal cutoff (92% sensitivity, 100% specificity).
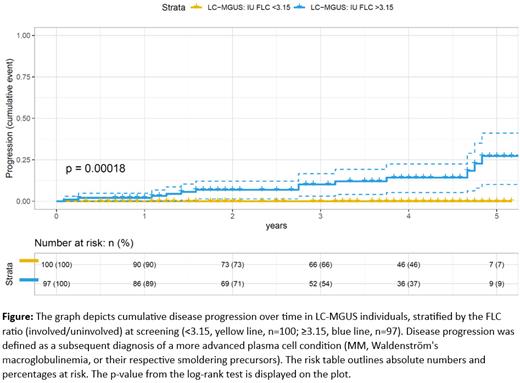
Of the 254 individuals randomized to active follow-up within the iStopMM study, baseline diagnosis distribution was 223 (87.8%) LC-MGUS, 22 (8.7%) LC-SMM, 7 (2.8%) LC-MM, 1 (0.4%) solitary plasmacytoma, and 1 (0.4%) chronic lymphocytic leukemia. Among these, 123 (representing 88% of the screened study cohort) had FLC ratio <3.15 at screening, thereof 122 (99.2%) with LC-MGUS and 1 (0.8%) with solitary plasmacytoma. No disease progression was observed for individuals with FLC ratio <3.15 (n=100) over a median follow-up of 46 months. Conversely, disease progression occurred in 13/97 (13.4%) LC-MGUS with ≥3.15 FLC ratio (9 to SMM, 3 to MM, and 1 to smoldering Waldenströms macroglobulinemia) during median follow-up of 42 months (p=0.00018 vs FLC ratio <3.15) (Figure).
The linear mixed-effects model of 454 repeated FLC from 128 individuals with <3.15 FLC ratio at screening did not indicate significant change in FLC ratio over a median follow-up of 48 months.
Conclusions: In this study, we utilized NGF to confirm an underlying clonal plasma cell population in BM of individuals with LC-MGUS and evaluate its association with involved/uninvolved FLC ratio. Clonal plasma cells were detected in a minority of LC-MGUS (38.1%), for which FLC ratio was found to be highly predictive. An FLC ratio cutoff of 3.15 optimally discriminated based on clonal plasma cell presence, and we found no evidence of progressive disease in LC-MGUS below this cutoff during follow-up. Altogether, the more stringent FLC ratio cutoff can assist in determining the likelihood of an underlying clonal plasma cell population in LC-MGUS and may, as such, help to guide the management of LC-MGUS.
Disclosures
Harding:Bindingsite ltd.: Current Employment, Membership on an entity's Board of Directors or advisory committees. Kristinsson:Celgene: Research Funding; Amgen: Research Funding.