Background: With the risk of thrombosis elevated in cancer patients, there is a need to find treatments that can regulate clotting abnormalities during this disease. The immune system has become a target for treating cancer-associated thrombosis (CAT). Notably, the macrophage, a key immune cell, has been shown to play a role in mediating cancer progression and thrombosis. A regulator of the coagulation process, urokinase plasminogen activator (uPA) also mediates cancer progression. uPA regulates blood clot breakdown through fibrinolysis. Monocytes have receptors for uPA. In addition, upon stimulation, they can release tumor necrosis factor alpha (TNF-α), a prothrombotic cytokine. Furthermore, macrophages can release higher quantities of tissue factor upon polarization through interleukins (IL-4 and IL-13). However, no known studies show the macrophage phenotypes' (pro-inflammatory or anti-inflammatory) effects on clot formation, structure, and fibrinolysis. Given that uPA has a role in cancer progression, monocytes contain receptors for uPA, and macrophage phenotype influences tissue factor expression, there is a need to determine how macrophage phenotype and components of the urokinase system can mediate clot formation, structure, and resolution.
Aim: We aim to elucidate the biochemical and structural mechanism(s) by which both pro-inflammatory (M1) and anti-inflammatory (M2) macrophages can induce CAT in the presence of the urokinase system.
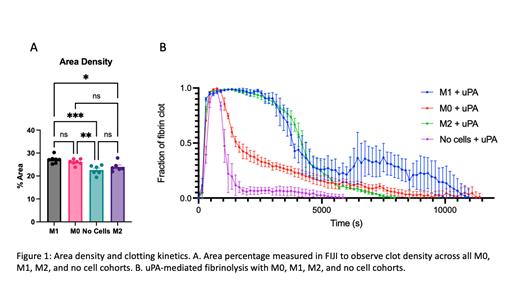
Methods: Human monocytes, THP-1 cells, were differentiated into M0, then attenuated into M1 or M2 macrophages using LPS or LPS+PGE2, respectively. Human plasma was coincubated with the respective macrophage phenotypes, and clot formation was tracked over time by collecting turbidity measurements using a microplate reader (n=2-3). This same coincubation experiment was also conducted with the addition of uPA to track fibrinolysis kinetics (n=3). Confocal microscopy was used to characterize clot structure; the fibrin network was labeled with a 488 nm fluorophore. Images (n=6-7) were measured for area percentage in FIJI to assess clot density.
Results: Clotting kinetics showed that all macrophage phenotypes resulted in a >1.4-fold faster onset of clotting compared to controls without cells (p<0.002). The faster onset of clotting corresponded to an increased fibrin area density relative to no cells, where proinflammatory M1 macrophages had the most pronounced effect (p<0.001) (Figure 1A). Fibrinolysis triggered by uPA indicated that M1 and M2 attenuated macrophages delayed the time to 50% lysis relative to M0 macrophages and no cells (Figure 1B).
Conclusion: Attenuation to the M1 and M2 macrophage phenotype promoted resistance to uPA-based lysis compared to M0 and no cell cohorts, suggesting that the change of phenotype can slow uPA-based lysis. Overall, all macrophage phenotypes accelerated clotting relative to controls without cells, which suggests macrophages can induce a hypercoagulable state leading to a denser fibrin network. We propose that the denser fibrin network is more resistant to fibrinolysis, thereby shedding light on a potential mechanism for the role of macrophages in immunothrombosis and CAT.
Disclosures
No relevant conflicts of interest to declare.