INTRODUCTION:
Avatrombopag is a thrombopoietin receptor agonist (TPO-RA) indicated for the treatment of severe thrombocytopenia in adult patients with chronic liver disease (CLD) who are scheduled for an invasive procedure and for chronic primary immune thrombocytopenia (ITP) in adult patients who do not respond to other treatments. In our health area, its use was approved in December 2020, although previously it could be used under individualized approval by the competent body in this matter.
OBJECTIVES:
The main objective is to report the experience of Avatrombopag in patients, from two third-level reference centers, diagnosed with chronic ITP who have failed other treatments, with CLD in the perisurgical setting and in myelodysplastic syndrome (MDS).
METHODS:
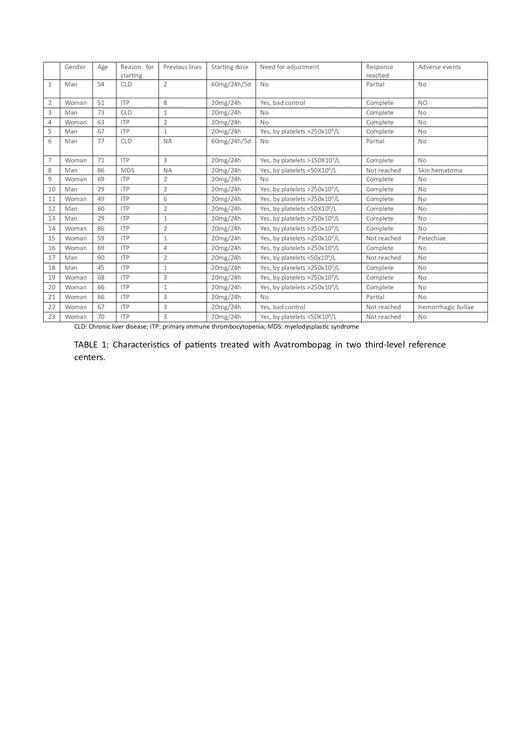
A total of 23 patients starting Avatrombopag from August 2022 to May 2023 were selected: 19 patients with chronic ITP, 3 patients with CLD prior to surgery and 1 patient with MDS. The data was collected in a data collection notebook.
RESULTS:
Of the patients who started with chronic ITP, 68% were women and 32% men, with a median age of 62 years (29-90). The median number of patients' previous lines was 2.63 (1-8). 100% of patients diagnosed with ITP had previously received corticosteroids and 42.10% had received another TPO-AR (15.78% had received both Eltrombopag and Romiplostim). The initial dose in all cases was 20mg/24h with 84.21% of patients requiring dose adjustment after the week of onset. In the 73.68% of the patients, platelet count >100x10 9/L (complete response) was achieved, in the 5.26% platelet count between 30x10 9/L and 100x10 9/L (partial response) and in the 21.06% of the patients there was no response, with an average platelet count of 172X10 9/L.
The 100% of the patients who started Avatrombopag for CLD in a peri-surgical setting were men. In one of them they started treatment with IVIg and platelet transfusion and in the other two patients in 1st line. The initial dose in 2 cases was 60mg/24h for 5 days, with no need for subsequent dose adjustment. The patient who started with the 20mg/24h dose also did not require dose adjustment. On 2 occasions the response obtained was partial and in one of them a complete response was obtained.
The patient with MDS who started treatment with Avatrombopag was in 1st line, to decrease the transfusional support of platelets, although without achieving response despite dose adjustments.
Tolerance was generally good, with a single patient presenting self-limited headache and a patient with mild asthenia. No thrombotic events were reported and there was a single spontaneous superficial skin hematoma, one patient with hemorrhagic bullae, and another patient with spontaneous petechiae. (TABLE 1).
CONCLUSIONS:
Avatrombopag is a second-generation TPO- RA with a good safety and efficacy profile in patients with chronic ITP and CLD prior to surgery. Oral administration and non-interference with food has allowed good tolerance in our patients, with a speed of action superior to that of other TPO-RA and has a widespread acceptance by the patients themselves, which ensures good adherence to it.
Disclosures
No relevant conflicts of interest to declare.