Background:Cancer therapy induced thrombocytopenia (CTIT) is a common complication of systemic treatment for cancer, both in solid tumors and hematologic malignancies. Some patients had persistent severe thrombocytopenia that failed to respond to thrombopoietin receptor (TPO-R) agonists such as eltrombopag, hetrombopag or recombinant human thrombopoietin (rhTPO). Avatrombopag, a second generation TPO-R agonist, has been approved for the treatment of thrombocytopenia in patients with chronic liver disease scheduled to undergo invasive procedures and primary chronic immune thrombocytopenia (ITP). Recent studies testified the effect of avatrombopag in ITP patients not responding to romiplostim and/or eltrombopag. In this study, we share the experience of the off-label use of avatrombopag in the management of CTIT with grade 4 thrombocytopenia, and assessed the efficacy as well as safety of the drug.
Methods: This study retrospectively evaluated patients with severe and refractoryCTIT treated at Qilu Hospital of Shandong University between 2021-2023 who received avatrombopag. Severe and refractoryCTIT met all of the following conditions: platelet count <25 × 10 9/L as grade 4 thrombocytopenia; previously unresponsive to rhTPO or TPO-R agonists other than avatrombopag. Patients' baseline characteristics were summarized in Table 1, including tumor types, dosing and duration of avatrombopag administration, and response at 4 weeks. The primary outcome was achievement of a response measured through (1) the proportion of patients with platelet count ≥50×10 9/L and increased by ≥ 100% above baseline at 4 weeks after medication and throughout the medication, (2) the time from avatrombopag initiation to first achievement of platelet count ≥50 × 10 9/L and increased by ≥ 100%, (3) the median maximum platelet count throughout avatrombopag administration. Safety was assessed by monitoring treatment emergent adverse events such as venous thromboembolism (VTE) development during treatment.
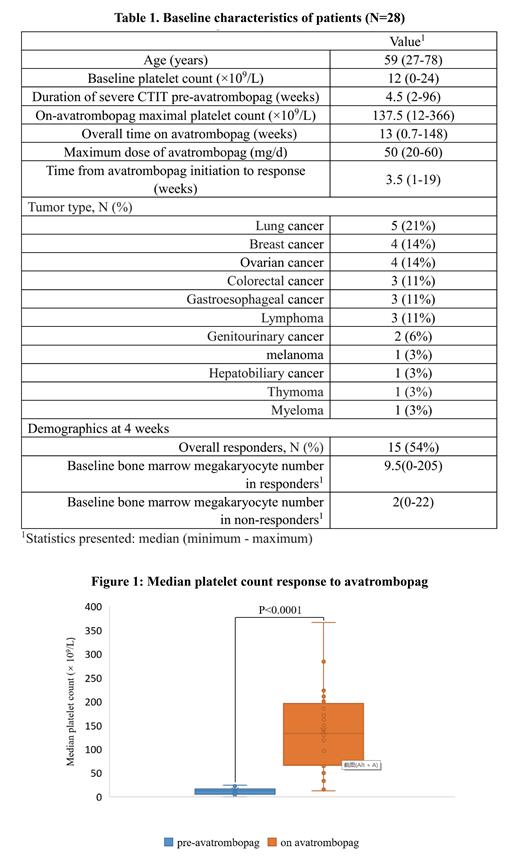
Results: A total of 28 patients were included with mean age 59 years (range 27 to 78). The median duration of severe CTIT pre-avatrombopag was 4.5 weeks (range 2 to 96). The overall median time on avatrombopag support was 13 weeks (range 0.7 to 148). The median maximum dose was 50 mg QD (range 20 mg QD to 60 mg QD). Treatment with avatrombopag led to significantly higher median platelet count compared with baseline (137.5 × 10 9/L vs. 12 × 10 9/L; p < 0.0001) (Figure 1), and the response rate was 86%. The median time to response was 3.5 weeks (range 1 to 19). Of the total 24 solid tumor patients, 22 (92%) achieved a platelet response. Fifteen of the 28 patients (54%) met the response criteria at 4 weeks. With respect to disease subgroup, patients with breast cancer on avatrombopag had higher platelet count compared to ovarian cancer subgroup (p = 0.048), while the duration of severe CTIT pre-avatrombopag in the two groups was similar. At 4 weeks, the response group showed an increased tendency in the median number of bone marrow megakaryocytes in smear at baseline than the non-response group (9.5 vs. 2; p = 0.069). In assessing incidence of adverse events, 1 patient were noted to be on anticoagulation for a documented VTE at six days post-avatrombopag discontinuation.
Conclusions: Avatrombopag was effective and safe in patients with severe and refractory CTIT. Future, prospective study is needed to investigate the efficacy of TPO-R agonists in patients with severe and refractory CTIT.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Avatrombopag is an orally bioavailable, small molecule thrombopoietin receptor agonist that stimulates proliferation and differentiation of megakaryocytes from bone marrow progenitor cells, resulting in an increased production of platelets. Avatrombopag does not compete with thrombopoietin for binding to the thrombopoietin. In this study, we share the experience of the use of avatrombopag in the management of severe and refractory cancer therapy induced thrombocytopenia, and assessed the efficacy as well as safety of the drug.