Background: Paroxysmal Nocturnal Hemoglobinuria (PNH) is an ultra-rare (0.5-1.5 cases per million), acquired, life-threatening disease characterized by complement-mediated hemolysis and thrombosis (Parker et. al. NORD 2023). PNH-related symptoms are multifactorial and may negatively impact cognitive function, including a difficulty in thinking clearly or “brain fog” (Daly et. al. JPRO 2021). Pegcetacoplan (PEG), a C3 inhibitor therapy, has been assessed in its efficacy for treating PNH and improving hemoglobin (Hb) levels in clinical trials; however, there is limited evidence on the effect of PEG treatment on cognitive function in individuals living with PNH in a real-world setting. Notably, the Patient-Reported Outcomes Measurement System (PROMIS) is a validated system of questionnaires used to reliably assess patient reported outcomes, including cognitive function. The minimal important change (MIC), the smallest change in score that patients perceive as important, is between a 2-6 improvement in T-score for PROMIS measures (Terwee et. al. Qual. Life Res. 2021).
Objectives: Perform an exploratory assessment of patient reported outcomes related to cognitive function for PNH patients treated with PEG and assess Hb level changes in a real-world setting.
Methods: Starting in January 2022, OPERA, a centrally recruited, nationally representative study (institutional review board approved) enrolled US patients with PNH, age ≥18, who were prescribed PEG by a licensed medical professional. Hb was reported by the site (healthcare provider verified) at baseline and was patient reported during monthly calls in the follow-up period. Hb analysis only included patients with no reported transfusions during PEG treatment, who reported both a baseline and ≥1 follow-up Hb value. To assess changes in cognitive function over time, participants completed the PROMIS Short Form v2.0 - Cognitive Function Abilities Subset 8a questionnaire online at baseline, 3 months, and 6 months following initiation of PEG treatment. Responses were provided on a 5-point Likert scale, and the sum of raw scores for survey responses were standardized to a T-score metric according to the PROMIS Cognitive Function Scoring Manual. The difference between T-scores at baseline and 3- or 6-months was determined. An item-level analysis was performed to determine the percent of patients that had a ≥1-level improvement (on Likert scale) at 3- and 6-months for individual PROMIS questions. Given disease rarity, a small sample size was expected.
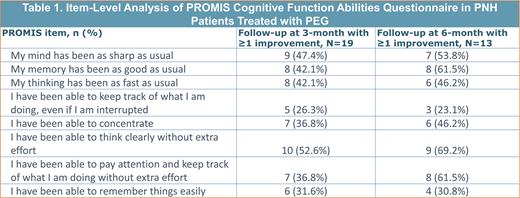
Results: Over 18 months, OPERA enrolled 62 patients with PNH, mean ± SD age of 45.3 ± 16.4, 53.2% female. Of 39 participants meeting Hb analysis criteria, mean ± SD Hb at baseline was 8.8 ± 1.8 g/dL and the latest reported Hb was 12.1 ± 2.1, with a median (IQR) follow-up period of 7.2 (6.1) months since initiating treatment. A total of 19 eligible participants had completed the PROMIS questionnaire at 3 months, and 13 participants had completed the questionnaire at 6 months. The mean ± SD improvement in T-score for PROMIS cognitive function was 4.0 ± 9.2 at 3 months and 4.2 ± 8.1 at 6 months. The majority of patients reported improvements to the question “I have been able to think clearly without extra effort” with 52.6% having a ≥1-level increase at 3 months and 69.2% at 6 months. The largest shift in patients reporting a ≥1-level increase across time were responses to “I have been able to pay attention and keep track of what I am doing without extra effort”; 36.8% reported a ≥1-level improvement at 3 months and 61.5% at 6 months. Of patients with eligible Hb analysis and follow-up PROMIS scores, patients with a MIC in PROMIS scores also had increased Hb from baseline levels (n= 7, mean ± SD Hb change from baseline 3.3 ± 1.3 g/dL).
Conclusions: This real-world study indicates that US adults with PNH being treated with PEG have increased Hb levels, and that cognitive function improves at 3 months and 6 months following initiation of treatment. Confirmatory clinical trials may be warranted to determine the relationship between improved cognitive function and Hb and to assess the efficacy of PEG treatment for improving these measures.
Disclosures
Fishman:Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Min:Apellis Pharmaceuticals, Inc.: Current Employment. Arnett:Boston Strategic Partners, Inc.: Current Employment. Shenoy:Boston Strategic Partners, Inc.: Current Employment.