Background
Mantle cell lymphoma (MCL) is a highly aggressive B-cell lymphoma characterized by the translocation t(11;14)(q13;q32) which triggers the dysregulation of the cyclin D1 by placing its coding gene CC1ND1 under the control of an immunoglobulin promoter. One of the major consequences of this molecular event is the disturbance of the cyclin D/Rb pathway leading to Rb inactivation and activation the elongation factor E2F1 mainly implicated in cell cycle progression. The mainstay of MCL therapies remains the chemo-immunotherapy followed by autologous transplantation but recently several novel therapies have been developed such as BTK inhibitors, Venetoclax, CDK4/6 inhibitors and more recently CAR-T therapies. However, patients with aggressive forms of MCL exhibit low response rates, requiring the identification of novel therapeutic targets.
Materials and Methods
We have previously generated an MCL cell line (UPN-1) from a patient with a blastoid variant of MCL presenting unique morphological and cytogenetics characteristics (M'kacher et al, Oncogene 2003). This cell line has been shown to harbor a TP53 mutation and a highly increased sensitivity to radiation despite the absence of functional p53. The molecular basis of this phenomenon has not been explored. In this work, we further characterized UPN-1 cell line using the Clarium S human microarray which has been compared to the gene expression profile of other existing MCL cell lines (GRANTA-519, JEKO-1, JVM-2, MAVEK-1, MINO, REC-1, SP-49, SP-53, Z-138). After mathematical correction, a supervised analysis was performed between triplicate samples of UPN-1 and a group constituted with all other MCL cell lines. We have then set up in vitro assays to determine the effect of a direct E2F1 inhibitor on the proliferative potential of UPN-1.
Results
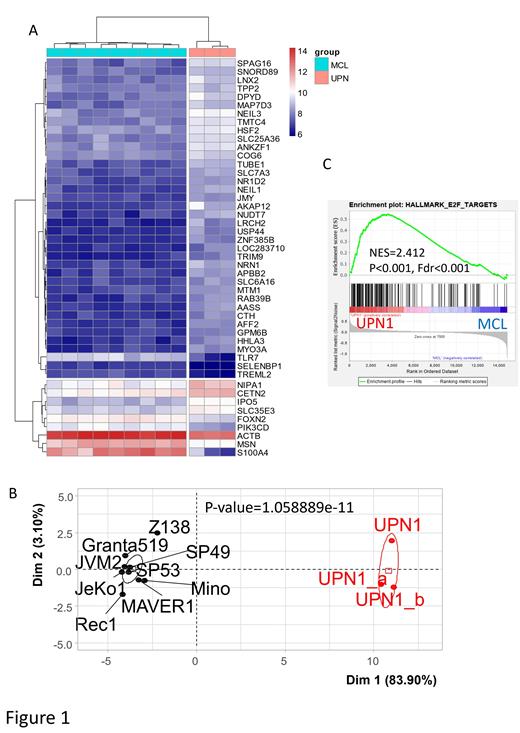
The comparative transcriptome analyses revealed a list of 47 genes specifically upregulated in UPN-1. Unsupervised classification allowed to discriminate UPN-1 samples from other MCL cell lines based on the expression of these 47 genes. These data suggested a specific expression profile of UPN-1 as compared to other cell lines. Among the upregulated genes, E2F1 elongation factor was found to be highly upregulated in UPN-1 as compared to other MCL cell lines along with positive enrichment of E2F targets which are mainly implicated in cell cycle progression (Figure 1). Interestingly, UPN-1 has a low level of BCL-2 as compared to other MCL lines that could explain its radio sensitivity. However, other signaling pathways involved in the inhibition of apoptosis were found to be present in UPN-1 by geneset enrichment analysis. The expression of CDKN1A (p21), major cell cycle regulator and known as a marker of aggressive MCL, was found to be low in UPN-1. A repression of immune response pathway such as NFKB with low expression of NFKBIA was identified. Finally, the expression of PIK3CD which encodes for a therapeutic target in MCL (such as PI3KDelta inhibitor Idelalisib) was also significantly repressed in UPN-1 as compared to nine other MCL cell lines. To evaluate the effect of the direct inhibition of E2F1, we treated UPN-1 cells with a targeted E2F1 inhibitor 5‘-Deoxy-5‘-(methylthio)adenosine (MTA) which is a protein methylation inhibitor. MTT assays revealed a 50% reduction of proliferation at + 4 hours in comparison to the vehicle-treated conditions (P<0.0001).Annexin assays showed an increase in early apoptosis (36.6 %) at + 8 hours with subsequent rise in late apoptotic cells (64.5 %) observed after 24 hours of treatment. Interestingly, qRT-PCR analyses of MTA-treated cells exhibited a decrease in the expression of downstream factors associated with E2F1, such as RAD51, BRCA1, CDK2, CCNB2, RANBP1.
Conclusions
We report here for the first time the comparative transcriptome analysis of the unique aggressive blastoid MCL line UPN-1 as compared to other MCL lines described. This cell line exhibited a major upregulation of E2F1 expression and a down regulation of the cell cycle inhibitor CDKN1A(p21). The potential effects of the direct inhibition of E2F1 using the targeted inhibitor MTA demonstrated a reduction in cell proliferation and induction of apoptosis, suggesting the possibility of associating clinically acceptable E2F1 inhibition strategies to other current therapies.
Disclosures
No relevant conflicts of interest to declare.