INTRODUCTION
PAX5::JAK2 is a gene fusion that drives a subset of Philadelphia chromosome-like acute lymphoblastic leukemia (Ph-like ALL) and confers aggressive disease, poor prognosis and poor response to treatment. PAX5::JAK2 is present in approximately 12% of Ph-like ALL cases. Targeted therapy is urgently required to improve outcomes. We evaluated the efficacy of JAK inhibitor ruxolitinib (RUX) and proteosome inhibitors carfilzomib (CFZ) and bortezomib (BTZ) as monotherapy and in combination against PAX5::JAK2 transfected Ba/F3 cells in vitro. Downregulation of proteosome genes via reduction in STAT3 phosphorylation is a shared mechanism of RUX and proteosome inhibitors. Given that the PAX5::JAK2 fusion results in increased STAT3 phosphorylation driving proliferation, it was proposed that the combination of proteosome inhibitors and RUX would demonstrate synergy in inhibiting proliferation of PAX5::JAK2 driven leukemic cells.
METHODS
Mouse derived Ba/F3 cells, an IL-3 dependent B-ALL model, were transfected with the PAX5::JAK2 fusion and demonstrated IL-3 independence. We applied, as single treatments, differing concentrations of RUX and the proteosome inhibitors CFZ and BTZ to PAX5::JAK2 transfected Ba/F3 cells and two control cell lines - IL-3 dependent empty vector Ba/F3 cells and an IL-3 independent KG1a myeloid cell line. Differing concentrations of CFZ and 200nM of RUX were then applied in combination. 200nM of RUX was selected because it is a dose at which the drug has minimal effect as a single agent. Inhibitors were applied to cells for 72 hours then percentage of live and dead cells at each concentration was ascertained by flow cytometry using Annexin V and LIVE/DEAD fixable aqua dead cell stain. Downstream STAT5 and STAT3 activation in basal state, and as a response to treatment, was assessed using phosphoflow. We demonstrated mechanisms of growth inhibition via reduction in STAT5 and STAT3 phosphorylation by the inhibitors.
RESULTS
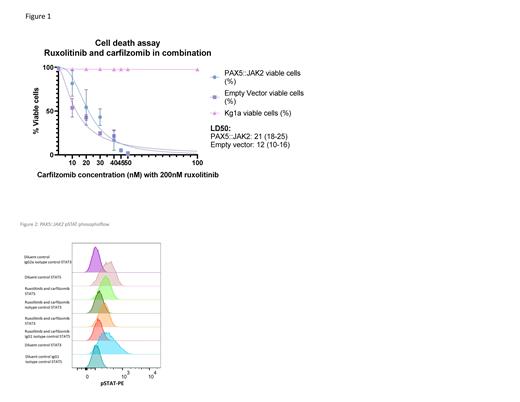
RUX, CFZ and BTZ were all effective against PAX5::JAK2 transfected Ba/F3 cells with a median lethal dose (LD50) of 514nM (CI 498-530nM) for RUX, 38nM (CI 36-39nM) for CFZ and 19nM (CI 18-20nM) for BTZ. RUX had a variable effect on IL-3 dependent empty vector control cells which also demonstrate JAK/STAT activation, but no effect on the IL-3 independent KG1a cells. CFZ had a lesser effect on empty vector control cells with a LD50 of 59nM (CI 58-60nM) and no effect on KG1a control cells. BTZ had a significant effect on both control cell lines, predicting off-target toxicity and consequently was not applied in combination with RUX. RUX and CFZ in combination demonstrated an LD50 of 21nM (CI 18-25nM) (see figure 1) which is significantly lower than the LD50s for either agent used as monotherapy. Combination index for RUX and CFZ at 45nM was 0.80 and at 50nM was 0.49, suggesting moderate synergy. Phosphoflow demonstrated reduced pSTAT5 with the application of RUX and CFZ to PAX5::JAK2 transfected Ba/F3 cells with mean fluorescence intensity (MFI) reducing from 351 to 285 in inhibitor treated cells, while for STAT3 MFI reduced from 396 to 249. (see figure 2) There was no effect on MFI for control cell lines. This demonstrates that the mechanism for growth inhibition is inhibition of JAK/STAT signaling due to the reduction in pSTAT3 and pSTAT5.
CONCLUSIONS
We showed in vitro that RUX, CFZ and BTZ are all potent against the high risk PAX5::JAK2 fusion. RUX and CFZ in combination demonstrated moderate synergy against PAX5::JAK2 and inhibited proliferation through their effect on JAK/STAT pathways. The RUX/CFZ combination, drugs widely available in clinical practice, is particularly promising given the apparent synergistic effect on limiting leukemic cell growth. Such treatments warrant clinical trials for management of the poor prognostic group of Ph-like ALL with PAX5::JAK2, given that they may enhance efficacy and lessen toxicity. Clinical trials have already demonstrated efficacy and safety of RUX and CFZ as single agents in combination with chemotherapy for the treatment of B-ALL which indicates the feasibility of combining the two agents clinically in Ph-like ALL.
OffLabel Disclosure:
Yeung:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding.
Carfilzomib and bortezomib use for acute lymphoblastic leukemia.