Introduction
In recent years, the treatment of B-cell acute lymphoblastic leukemia (B-ALL) has evolved tremendously resulting in improved outcomes in patients with relapse/refractory (r/r) B-ALL and patients with measurable residual disease (MRD+). One such novel therapy is the CD3/CD19 bispecific antibody blinatumomab. There is however limited data on the outcomes and treatment options for B-ALL patients who relapse after blinatumomab was given as salvage therapy.
Methods
This IRB-approved study retrospectively reviewed the outcomes of all adult patients (≥18 years old) with B-ALL treated with blinatumomab at our center, Singapore General Hospital. The primary aim was to describe the incidence, risk factors and survival outcomes of B-ALL patients who relapse after blinatumomab salvage therapy.
Results
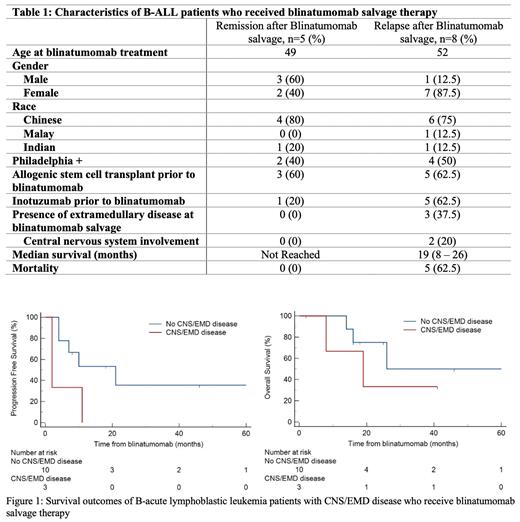
Between January 2017 to December 2022, a total of 27 patients were treated at our center with blinatumomab. Blinatumomab was used as salvage therapy in 13 patients and for MRD+ disease after front-line therapy in 7 patients. Characteristics of the 13 patients who received blinatumomab salvage therapy are shown in Table 1. Eight patients had received an allogenic hematopoietic stem cell transplant (alloHSCT) and 6 patients had received inotuzumab, a CD22 antibody-drug conjugate, prior to blinatumomab salvage. The median number of lines of therapy before blinatumomab salvage was 3 and the median number of cycles of blinatumomab administered was 4. All but 1 patient achieved MRD negativity after the prescribed number of blinatumomab cycles. Eight (61.5%) of 13 patients relapsed after blinatumomab salvage therapy. Median progression free survival (PFS) in this group of patients was 7 months (IQR 2-11) and overall survival (OS) of 19 months (IQR 8-26). Six (75%) of these 8 patients proceeded to receive salvage therapy with inotuzumab. Majority (5 of 6) achieved MRD negativity and 2 patients subsequently underwent alloHSCT and CAR-T therapy respectively. Unfortunately, all 6 patients eventually relapsed. Of note, 3 patients had received inotuzumab prior to blinatumomab salvage.
We observed that patients (n=3) with extramedullary disease (EMD) (including central nervous system (CNS) involvement) at initiation of blinatumomab salvage therapy were associated with higher odds of both systemic and extramedullary relapse (p=0.014). These patients had inferior progression free survival (PFS) as compared to patients without EMD/CNS involvement, median PFS 2 months (95% CI 2 - 11) vs 21 months (95% CI 4 - 21), p = 0.041 (Figure 1). A trend towards inferior overall survival was also observed though statistical significance was not demonstrated, median OS 19 months (95% CI 8 - 19) vs 26 months (95% CI 14 - 26).
Conclusion
EMD/CNS involvement prior to blinatumomab salvage therapy was associated with higher risk for systemic and EMD/CNS relapse. Patients with B-ALL who relapsed after blinatumomab salvage could be successfully rescued by inotuzumab regardless of prior inotuzumab use. These groups of patients however represent very-high risk groups with inferior survival outcomes. Novel treatments and better sequencing of treatments may improve outcomes of these patients.
Disclosures
No relevant conflicts of interest to declare.