Introduction: Standard induction chemotherapy in newly diagnosed acute myeloid leukemia (AML) includes a combination of standard-dose cytarabine and an anthracycline (idarubicin [IDA], daunorubicin [DNR], or mitoxantrone [MXR]). Anthracycline selection is still not standardized, and the impact of anthracycline selection on survival and response in patients with adverse genetic risk is of interest. The study aimed to retrospectively compare the effectiveness of induction regimens with respect to anthracycline preference.
Methods: The study population was recruited from the Turkish AML Registry project database based on documentation of newly diagnosed AML and induction chemotherapy with IDA, DAU, or MXR in combination with cytarabine. Patients diagnosed before 31 August 2022 were received typical “7 + 3” induction chemotherapy containing 3 days of IDA, DAU, or MXR.
Results: The study included 317 newly diagnosed AML patients (median age 51; range, 18 - 79; 55.2% male). Of the patients, IDA was administered in 266 (83.9%), DNR in 42 (13.2%), and MXR in 9 (2.8%). The three anthracycline treatment groups were similar between age, gender, type of AML by the World Health Organization (WHO), and risk classification by 2017 European LeukemiaNet (ELN) when evaluated according to the baseline disease characteristics. At the time of diagnosis, although the median white blood cell levels were 19 x 10 9/L (0.4 - 355) in the IDA, 7.8 x 10 9/L (0.7 - 357.2) in the DAU, and 5.5 x 10 9/L (0.6 - 34) in the MXR therapy group (p = 0.019); the hemoglobin and platelet levels and bone marrow blast percentage were similar between the treatment groups. FMS-like tyrosine kinase-3 internal tandem duplication ( FLT3-ITD) (p = 0.561) and Nucleophosmin 1 gene ( NPM1) (p = 0.430) mutation frequencies were also similar between treatment groups. Morphological complete remission (CR) was documented in 65% of all evaluable patients. Among the patient groups, CR was 67.7% for IDA, 50% for DNR, and 55.6% for MXR (p = 0.081).
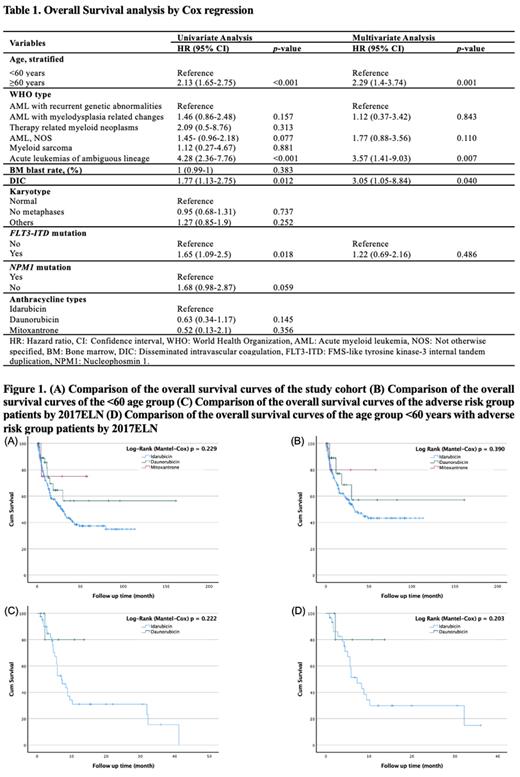
In univariate analysis (Table 1), significant predictors of overall survival (OS) were age ≥60 years (p <0.001), acute leukemias of ambiguous lineage (p <0.001), presence of disseminated intravascular coagulation (DIC) (p = 0.012), and FLT3-ITD mutation (p = 0.018); no significant interaction was noted between NPM1 mutation and OS (p = 0.059). Additionally, when idarubicin was taken as a reference, there was no meaningful relationship between anthracycline type and OS (p values for daunorubicin and mitoxantrone were 0.145 and 0.356, respectively). In multivariate analysis (Table 1), age ≥60 years (p = 0.001), acute leukemias of ambiguous lineage (p = 0.007), and the presence of DIC (p = 0.040) remained significant.
After a median follow-up of 29.8 months, 32.5% relapse cases were documented and similarly distributed among IDA (33.1%), DNR (31%), and MXR (22.2%) therapy groups (p = 0.771). During the same period, allogeneic hematopoietic stem cell transplantation (AHSCT) was documented in 10.9% and 26.2% of patients treated for IDA and DNR, respectively. No patient progressed to AHSCT in the MXR arm (p <0.001).
In the study, 142 (44.8%) deaths were reported, and there was a 48.5%, 26.2%, and 22.2% mortality rate between the IDA, DNR, and MXR treatment groups, respectively. There was no significant difference in OS by anthracycline type in both the study cohort and age group <60 years (Figures 1A and 1B). There was also no significant difference in OS in patients with the adverse genetic risk group and <60 years age group with adverse genetic risk, according to 2017ELN (Figures 1C and 1D). The results may have been influenced by the number of patients in the treatment groups of the retrospective study and by differences in fitness levels that needed to be measured systematically.
Conclusions: We are encouraged that our findings mostly agree with previously published studies and that IDA, DNR, and MXR offer comparable survival values in the research and the younger patient groups. In the future, the ever-changing treatment environment in patient groups with targetable mutations makes choosing the optimal anthracycline for induction chemotherapy in AML much more difficult.
Disclosures
No relevant conflicts of interest to declare.