Introduction: Relapsed/refractory acute myeloid leukemia (R/R AML) remains challenging to treat. Unfit patients receive either targeted therapy for actionable mutations or low-intensity chemotherapy, often a hypomethylating agent with venetoclax (HMA/ven). Aggressive regimens, such as a purine analog with high-dose cytarabine, are reserved for fit patients. A regimen using similar drug classes, comprised of cladribine (clad), low-dose cytarabine (LDAC), and venetoclax (clad/LDAC/ven), has demonstrated favorable efficacy and safety for frontline AML treatment in older patients, according to recent phase 2 studies [Kadia 2018 Lancet Hematology; Kadia 2022 Journal of Clinical Oncology]. Interestingly, monocytic differentiation is a proposed mechanism of venetoclax resistance, and monocytic leukemic stem cells rely on purine metabolism, thus suggesting unique cladribine sensitivity [Pei 2023 Cancer Discovery]. The utility of clad/LDAC with or without venetoclax in R/R AML is not well characterized, especially in today's evolving treatment landscape.
Methods: In this single-center retrospective cohort study, we report efficacy and safety outcomes of a salvage regimen using a clad/LDAC backbone in adult patients with R/R AML between 2019 and 2023. For induction, patients received cladribine 5 mg/m2 IV daily days 1 to 5 and cytarabine 20 mg/m2 IV daily days 1 to 10. A third agent, such as venetoclax at a target dose of 400 mg oral daily days 1 to 21, was added at the provider's discretion. Dose reductions were allowed based on performance status and organ function. Re-induction was considered if the bone marrow biopsy demonstrated inadequate response. Patients who responded received clad/LDAC-based consolidation or proceeded to allogeneic hematopoietic cell transplant (alloHCT).
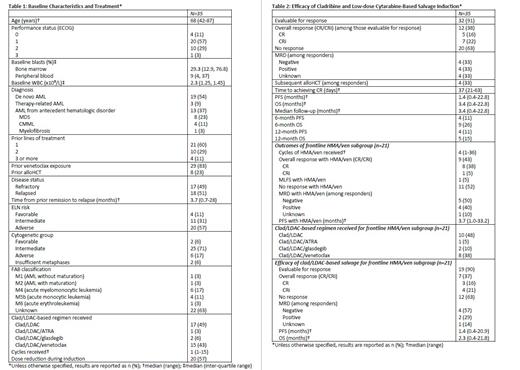
Results: A total of 35 patients received clad/LDAC-based salvage induction for R/R AML. Median age was 68 years old, and patients had a median of 1 prior line of therapy. Half of patients had secondary AML, and most had adverse risk disease. The most common regimen received was clad/LDAC (49%), followed by clad/LDAC/ven (43%). Among 32 patients evaluable for response, the overall response rate (ORR) was 38%, with complete response (CR) of 16% and complete response with incomplete count recovery (CRi) of 22%. The median progression-free survival (mPFS) was 1.4 months (range 0.4-22.8), and median overall survival (mOS) was 3.4 months (range 0.4-22.8). At 6 months, PFS was 11% and OS was 26%. At 12 months, PFS was 11% and OS was 15%. For responders, 33% achieved negative measurable residual disease (MRD), 33% proceeded to alloHCT, mPFS was 3.3 months (range 0.9-22.8), and mOS was 6.4 months (0.9-22.8). Mutational profiling of responders revealed ASXL1, CSF3R, NF1, SETBP1, and U2AF1 to be most the common. In a subgroup of 21 patients who had progressive or refractory disease with frontline HMA/ven, clad/LDAC-based salvage resulted in ORR 37%, CR 16%, CRi 21%, MRD negativity 57% among responders, mPFS 1.4 months (range 0.4-20.9), and mOS 2.3 months (range 0.4-21.8).
Clad/LDAC-based induction was well tolerated, with no cases of treatment-related mortality. Common adverse effects included febrile neutropenia (74%), grade 1 or 2 elevated bilirubin (49%), grade 1 transaminitis (48%), rash (43%), nausea/vomiting (37%), and grade 1 acute kidney injury (31%). One third of patients received clad/LDAC-based regimen in the outpatient setting.
Conclusions: Clad/LDAC and clad/LDAC/ven represent feasible salvage induction strategies for R/R AML that may be administered outpatient and associated with minimal toxicity. Favorable response rates occurred in a subgroup of patients who failed frontline HMA/ven, in which there is an unmet need.
OffLabel Disclosure:
Zhang:Rigel: Consultancy; Abbvie: Consultancy; Servier: Consultancy; Bristol Myers Squibb: Research Funding; Stanford University: Current Employment. Fakhri:BMS/Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genetech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab/Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; LOXO/Lilly: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Liedtke:Seagen: Other: Grants or contracts; Janssen: Other: Grants or contracts; Participation on a Data Safety Monitoring Board or Advisory Board; Caelum: Other: Grants or contracts; BMS: Other: Grants or contracts; Participation on a Data Safety Monitoring Board or Advisory Board; Allogene: Other: Grants or contracts; Adaptive: Other: Participation on a Data Safety Monitoring Board or Advisory Board; Abbvie: Other: Grants or contracts; Kite: Other: Participation on a Data Safety Monitoring Board or Advisory Board. Shomali:Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Mannis:BMS/Celgene: Consultancy; Astellas: Consultancy; Macrogenics: Honoraria; Agios: Consultancy; Abbvie: Consultancy; Genentech: Consultancy; Stemline: Consultancy.
Cladribine, low-dose cytarabine, and venetoclax for salvage AML induction