Introduction
Anti-CD19 chimeric antigen receptor T cells (CAR-T) have rapidly shifted the treatment paradigm for relapsed/refractory large B-cell lymphoma (LBCL) but produce durable complete response (CR) in only 30-40% of patients. In addition, CAR-T-mediated toxicities such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) remain a major concern. To investigate the relationships of circulating cytokines and chemokines on CAR-T outcomes, we applied specialized comprehensive cytokine panels to peripheral blood samples serially collected from LBCL patients undergoing treatment with anti-CD19 CAR-T cells.
Methods
LBCL patients were consented to our IRB-approved study and longitudinally followed for sample collection at pre-specified timepoints: prior to lymphodepleting chemotherapy (LD chemo), day 0 (day of [and prior to] CAR-T infusion), and days 3, 7, 10, 14, 21, and 90. MSD R-PLEX and U-PLEX assays were used to detect 47 cytokines and chemokines in plasma; vasoactive intestinal peptide (VIP) levels were quantified via a VIP-specific enzyme immunoassay. Mann-Whitney U test, Spearman's correlation coefficient, and a LASSO (least absolute shrinkage and selection operator) model were utilized to identify key cytokines contributing to critical clinical outcomes.
Results
Peri-CAR-T blood samples were collected from 24 LBCL patients, including one patient who received 2 infusions of an investigational allogeneic anti-CD19 product; all other patients received commercially available products, each associated with their distinct LD chemo dosing regimens. With median follow-up of 11.2 months, 54% patients achieved CR as best response, 50% experienced CRS (maximum Grade 2 in 25%), and 29% experienced ICANS (Grade ≥3 in 8%).
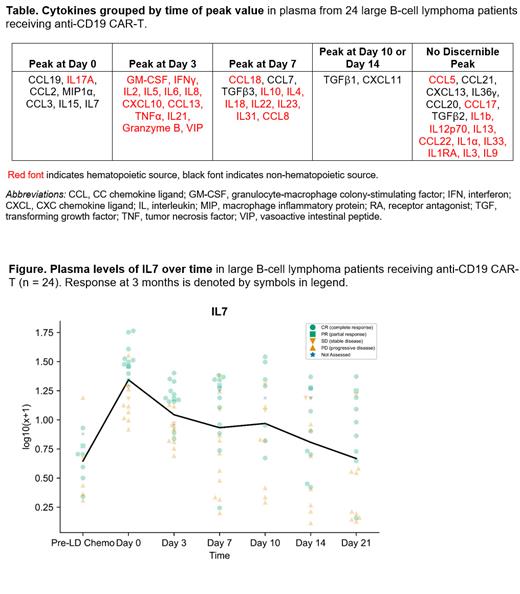
Most cytokines demonstrated characteristic peak patterns over time that were not dependent on whether cytokines were produced by hematopoietic cells ( Table). Among most cytokines with peak values at Day 0, the mean percentage change from Pre-LD Chemo to Day 0 increased as chemo intensity increased (i.e., fludarabine dose from 25 to 30 mg/m 2, and cyclophosphamide dose from 250 to 500 mg/m 2). Notably, levels of the non-hematopoietic cytokines IL7 and IL15 were highest at day 0, reflecting effects of LD chemo.
Using statistical models to filter relevant values, we developed a signature of 14 key cytokines that could be used to predict clinical response at 3 months. Half of these were stromal-produced cytokines from early timepoints: Cytokines positively correlated with CR or partial response (PR) included IL7 at Day 0 (Mann-Whitney U p<0.001) and Day 7 (p=0.044), and IL15 at Day 0 (p=0.016). Negatively correlated cytokines included IL4 at Day 3 (p=0.041), IL10 at Day 7 (p=0.027), CCL19 at Day 10 (p=0.017), and MIP1α at Day 14 (p=0.03). Intriguingly, several Type 1 T helper cell (Th1)-related cytokines with peaks at Day 3 or Day 7 (e.g., IL2, IFN-γ, TNF-α, and IL18) were not associated with response. Strong associations of timepoint-specific cytokine levels with patient outcomes are exemplified in the Figure: all patients achieving CR had IL7 levels above average on Day 0, while most patients (9/10) who failed to achieve CR had IL7 levels below average on Day 0. This same striking pattern was observed for IL15 at Day 0.
Similar analyses gauged which timepoint-specific cytokines were associated with CRS and ICANS. Of 14 values associated with CRS, 13 were from Day 3 or later, likely reflecting sequelae of CRS rather than representing predictive biomarkers. Pre-LD Chemo CCL18 was the exception, positively correlated with CRS (p=0.035). Only 5 cytokine values were associated with development of ICANS, 3 from Day 10 or later; IL7 at Day 0 (p=0.011) and Day 3 (p<0.001) was positively correlated with ICANS .
Conclusions
Comprehensive cytokine profiling revealed that early measurements of several stromal-produced cytokines can predict response in LBCL patients who receive CAR-T-most notably IL7 and IL15 levels on Day 0, prior to CAR-T infusion. Conversely, few early cytokine measurements were helpful in predicting CRS and ICANS, limited to CCL18 and IL7. Analyses are ongoing to characterize associations of patient characteristics, product differences, and circulating immune cell subsets with changes in cytokine profile over time. Further studies are warranted to validate our findings in a larger data set.
Disclosures
Waller:Allovir: Consultancy; CSL Behring: Consultancy, Research Funding; BMS: Research Funding; Secura: Research Funding; PartnersTherapeutics: Research Funding; Verastem: Consultancy, Research Funding; Cambium Medical Technologies: Current equity holder in private company, Other: Founder; NCI R01: Research Funding; Sanofi: Research Funding; ORCA: Research Funding; Novartis: Consultancy, Research Funding; CRISPR: Consultancy; Cambium Oncology: Current equity holder in private company, Other: Founder. Koff:Viracta Therapeutics: Research Funding; BeiGene: Consultancy.