Background: Advanced-stage follicular lymphoma (FL) and marginal zone lymphoma (MZL) represent incurable indolent B-cell lymphomas and perplex elders mainly. Chemoimmunotherapy can induce durable remissions; however, the side effects of chemotherapy remain a major source of concern for patients. Parsaclisib, a next-generation PI3K inhibitor, has demonstrated significant clinical activity in refractory or relapsed indolent B-cell lymphomas, including FL and MZL. This study investigated the efficacy and safety of a chemo-free regimen in patients with FL and MZL tumors. Here, we present preliminary results from a response-adapted study utilizing parsaclisib and rituximab as frontline therapy for FL and MZL (NCT05073250).
Methods: Eligible patients aged ≥18 years with previously untreated indolent B-cell non-Hodgkin lymphoma (NHL) of either FL grade 1-3a or MZL and stage III-IV disease (Patients with stage I-II FL and MZL who have received radiation therapy and subsequently relapsed were also eligible) were included in the study. Patients with active HIV, CMV, Hepatitis B or C infections, and autoimmune conditions requiring therapy were excluded. Patients received 6 28-day cycles of parsaclisib at a daily dose of 20mg during the first two cycles, followed by a reduced dose of 2.5mg daily for the subsequent four cycles; rituximab was administered at 375mg/m 2 weekly for 4 weeks, and then on day 1 of each cycle. All patients received PCP prophylaxis. Baseline FDG-PET and CT scans were performed, and additional scans were conducted after cycles 3 and 6 to assess treatment response. Patients achieving complete response (CR) after the 6 cycles discontinued therapy, while patients with partial response (PR) received another 6 cycles of the combination therapy. Non-responding patients were offered standard chemotherapy. The primary endpoint of the study is the overall rate of complete response (CR), with secondary endpoints including safety, objective response rate (ORR), duration of CR, and progression-free survival (PFS).
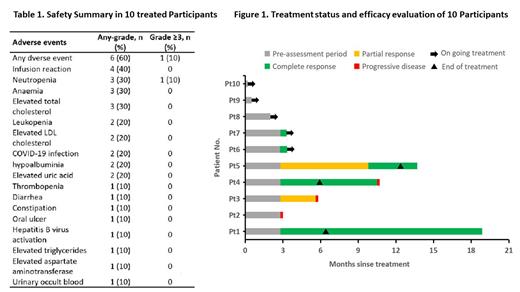
Results: As of the data cut-off on July 20, 10 patients were enrolled, with a median follow-up time of 10.2 months, and 7 patients were eligible for response evaluation. The median age of patients was 47 years (range 35-52), and three were older than 60. Fifty percent (5/10) of the patients were Follicular Lymphoma (FL), while the remaining 50% were the extra-nodal MZL of mucosa‐associated lymphoid tissue. Three patients had bone marrow involvement, and one had C-MYC/BCL-2 double expression. The Complete Response (CR) rate was 71.4%, with the FL cohort showing a CR rate of 75% (3/4) and the MZL cohort showing a CR rate of 66.7% (2/3). The Overall Response Rate (ORR) was 85.7% (6/7, with 3 cases in FL and 3 cases in MZL). The median duration of CR was 3.9 months (range 0.6-7.7), and the median Progression-Free Survival (PFS) was not reached. Five patients are still receiving treatment, two patients who completed treatment were followed up and remained in sustained complete remission. Among the 10 treated patients, treatment-emergent adverse events (TEAEs) occurred in 60% (n=6), and no serious adverse events (SAEs) were observed. The most common TEAEs were infusion reaction (40%, rituximab-related), neutropenia (30%), anemia (30%), and total cholesterol elevation (30%). Only one patient experienced grade 3 neutropenia, no grade 4-5 TEAEs occurred. Two patients experienced delayed medication due to COVID-19 infection.
Conclusion: The combination of parsaclisib with rituximab showed a deep and durable response, with a favorable safety profile and generally good tolerability in patients with previously untreated indolent B-Cell Lymphoma.
Disclosures
No relevant conflicts of interest to declare.