Introduction
Non-Hodgkin's lymphoma (NHL) is a globally prevalent cancer with a high mortality rate. Although advances in treatment have improved patient survival, patients with high International Prognostic Index (IPI) scores continue to face challenges. The CMOP regimen (cyclophosphamide, mitoxantrone, vincristine, and prednisone) has shown promising efficacy in peripheral T-cell lymphomas with an overall response rate of 84%, but there is a lack of evidence in other NHL subtypes. In this study, we present preliminary results of a CMOP±R (CMOP±rituximab) regimen for the initial treatment of patients with NHL. The study is registered at ClinicalTrials.gov (NCT 2300074073).
Aim
This prospective, single-arm, open-label, multicenter phase 2 clinical trial was designed to evaluate the efficacy and safety of a CMOP±R regimen including mitoxantrone hydrochloride liposome in patients with newly diagnosed NHL.
Method
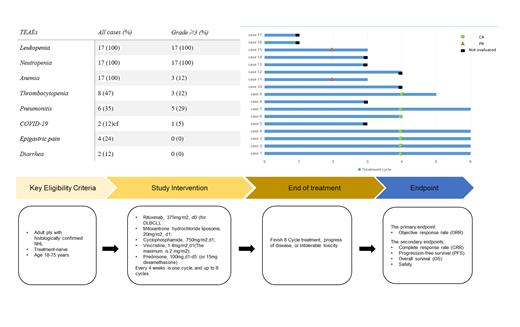
Adult patients with a confirmed diagnosis of NHL (predominantly DLBCL, MZL, FL, and AITL), ECOG performance status ≤1, normal organ and bone marrow function, and at least one measurable or evaluable lesion were enrolled in this study. Patients with significant cardiac dysfunction or uncontrolled cardiovascular disease were excluded. Treatment with the CMOP±R regimen was administered every 4 weeks for up to 6 cycles until disease progression or an intolerable toxic response. The primary endpoint was the objective response rate (ORR), which was assessed by the investigators. Secondary endpoints included duration of response (DoR), progression-free survival (PFS), overall survival (OS), and safety. Adverse events were graded using NCI Common Terminology Criteria for Adverse Events (CTCAE) 5.0. The study was approved by the Institutional Ethics Committee (IRB number: 2022021).
Results
From November 26, 2022 through May 19, 2023, 17 Chinese patients with various NHL subtypes who were eligible for enrollment were treated with the CMOP±R regimen. Nine of these patients were eligible for efficacy evaluation. Results showed that 100% of evaluable patients achieved an objective response and 66.7% achieved a complete response. Hematological toxicity was the most common treatment-related adverse reaction, with leukopenia (100%) and neutropenia (100%) being the most common grade ≥3 toxic reactions.
Conclusions
The CMOP±R regimen using mitoxantrone hydrochloride liposome showed efficacy in the initial treatment of NHL with acceptable and manageable adverse effects. The role of this regimen in the treatment of NHL warrants further exploration.
Disclosures
No relevant conflicts of interest to declare.