Introduction:
Plasmablastic myeloma (PBM) and plasmablastic lymphoma (PBL) are rare hematologic neoplasms that are pathologically difficult to distinguish. PBM represents less than 5% of all myelomas and is characterized by neoplastic proliferation of atypical plasmablasts in the bone marrow and extramedullary sites. Immunophenotypically, the neoplastic cells lack CD45 expression and exhibit plasma cell markers (CD38, CD138), with MYC overexpression and high proliferative index. PBLs are commonly seen in immunocompromised patients and account for ~2% of all HIV-related lymphomas. Histologically, they resemble diffuse large B cell lymphoma (DLBCL), but have a distinctly immunoblastic or plasmablastic morphology. Similar to PBMs, the neoplastic cells of PBLs are positive for plasma cell markers but negative for conventional B-cell markers (CD20, PAX5). Clinically, plasmablastic neoplasms respond poorly to therapy and have an aggressive clinical course, with median overall survival in some studies ranging from 8 to 15 months. There is no established standard of care for these disorders, and further characterization of this complex patient population is needed.
Methods:
Patients were identified from the pathology database at Los Angeles General Medical Center and Norris Comprehensive Cancer Center. Patient demographics, treatment, and clinical outcomes were collected for each patient. Overall survival was calculated for the total cohort, as well as patient subgroups including disease type, and HIV status.
Results:
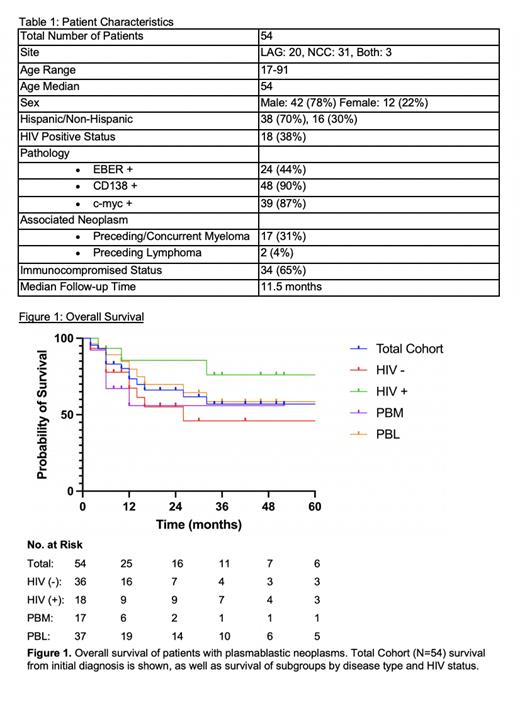
We retrospectively identified 54 patients diagnosed with plasmablastic neoplasms including PBM (31%, N=17) and PBL (63%, N=34) between January 2017 and May 2022. The median age at diagnosis was 54 years (range 17-91 years) and 78% were male. Hispanic patients represented 70% (N=36) of our total study population. Predisposing immunosuppression was identified in 65% (N=34), including 33% (N=17) on immunosuppressive drugs and 52% (N=27) with non-iatrogenic causes; 38% (N=18) of patients were HIV-positive. Pathologic analysis showed EBER positivity of 44% (N=24), CD138 positivity of 90%, and c-myc positivity of 87%. The most common initial regimens were EPOCH (17%, N=9) and V-EPOCH (33%, N=18). There was no superiority when comparing V-EPOCH versus all other treatment regimens. Seven patients received subsequent consolidation with high-dose chemotherapy followed by autologous hematopoietic transplant. Seven patients were lost to follow-up prior to initiating therapy or evaluating treatment response. Of the patients followed, 11% never started first line therapy, 38% had primary refractory disease, 34% achieved complete response, and 17% relapsed after 6 months of starting therapy. Overall survival (OS) for the total cohort was 73% at 12 months and 60% at 24 months. OS at 12 months varied between different subgroups: PBL (80%), PBM (56%), HIV-positive (86%), and HIV-negative (67%). Although HIV-positive trended towards a better OS than the HIV-negative cohort, it was not statistically significant (p=0.11).
Conclusions:
Plasmablastic neoplasms remain a heterogeneous group of diseases with a historically poor prognosis. Our PBL patients showed an improved OS at 12 months of 80% compared to the SEER database PBL OS of 56%. This improvement in survival could derive from ethnic variations in tumor biology in this predominantly Hispanic cohort, which is consistent with the improved OS in Hispanic patients seen in the SEER database. Other contributing factors to consider are differences in stage and co-morbidities, increased use of intensive therapies such as V-EPOCH and autologous stem-cell transplant, success of salvage therapies, and improved immune status from treatment of underlying HIV. Further studies are needed to improve therapeutic targets for this patient population.
Disclosures
No relevant conflicts of interest to declare.