Plasmablastic lymphoma (PBL) is an aggressive subtype of B-cell Non-Hodgkin lymphoma (B-NHL) with poor outcomes. PBL occurs more commonly in HIV-positive or immunocompromised patients, but it can also occur in immunocompetent patients. Despite anthracycline-based induction therapy, PBL is associated with chemotherapy resistance and early disease relapse, yielding a median overall survival of 7 to 15 months (Castillo JJ, et al. Blood. 2015). The advent of novel therapies altered the therapeutic landscape for many common subtypes of B-NHL, but progress in PBL is lacking due to the rarity of this disease and the lack of effective therapeutic targets. While PBL does not express many common B-NHL markers (e.g. CD19 or CD20), it does highly express B-cell maturation antigen (BCMA). However, data regarding the efficacy of therapies targeting BCMA in PBL is limited. Herein, we report two cases of relapsed/refractory PBL treated with the BCMA-targeting bispecific antibody teclistamab.
Patient 1 was a 33 year old male without significant comorbidities when he was diagnosed with Stage IIE PBL after presenting with a chest wall mass. He was treated with six cycles of dose-adjusted EPOCH-V, followed by radiation to the involved region and a BEAM autologous hematopoietic stem cell transplant. Post-transplant imaging showed him to be in a CR on day 30 PET, but he developed recurrent PBL on his day 100 PET exam with osseus lesions. He was then treated with daratumumab and ICE chemotherapy for four cycles, with an initial resolution of osseus lesions. However, he developed relapsed disease with perilymphatic lung nodules. His PBL was confirmed to express BCMA and he was started on teclistamab monotherapy. Following one cycle of weekly teclistamab (1.5mg/kg), his post-treatment PET imaging demonstrated a complete response. He then underwent a consolidative allogeneic hematopoietic stem cell transplant from a matched unrelated donor. As of this report, he remains in a complete response based on his 30 day PET restaging imaging after transplant and without clinical evidence of disease more than 90 days after transplant.
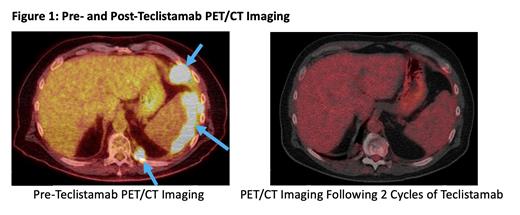
Patient 2 was a 60 year old male with medical history of comorbid CAD, stage IV chronic kidney disease, chronic back pain requiring opioid treatment, and peripheral arterial disease when he was diagnosed with Stage IV PBL after he was found to have mass-like thickening of his descending colon on a CT scan. He underwent partial colectomy, with pathology revealing PBL and his post-operative staging PET/CT scan noting osseus, splenic, and hepatic lesions consistent with advanced stage disease. He was initially treated with DA-EPOCH for six cycles with intrathecal methotrexate, which yielded an initial complete response, though he relapsed four months after completing therapy. He was then treated with gemcitabine, oxaliplatin, and daratumumab but progressed after eight cycles on treatment with abdominal and perisplenic masses confirmed on biopsy to be PBL He was then initiated on teclistamab, which was continued weekly. His treatment course was complicated by a viral pneumonia from which he recovered with IVIG and supportive measures. Following 2 total cycles of therapy, his repeat PET/CT imaging was consistent with a complete response (Figure 1), and he remains on weekly teclistamab therapy with no evidence of disease to date.
The encouraging responses noted in our two cases illustrate the therapeutic potential of targeting BCMA using teclistamab in PBL. In our cases, teclistamab was a well tolerated therapy, even in our patient with significant comorbidities. This report provides a foundation for future prospective, multicenter clinical trials to evaluate the efficacy and safety of teclistamab in the treatment of relapsed and refractory PBL.
OffLabel Disclosure:
Gurbuxani:AbbVie: Consultancy; Jazz Pharmaceuticals: Consultancy; UpToDate: Patents & Royalties: Royalties for contributions to various topics. Riedell:Roche: Research Funding; Nkarta: Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy; CRISPR Therapeutics: Research Funding; Janssen: Consultancy; Tessa Therapeutics: Research Funding; Xencor: Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nurix Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sana Biotechnology: Consultancy; Kite/Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; MorphoSys: Research Funding; Nektar Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Fate Therapeutics: Research Funding; Intellia Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CVS Caremark: Consultancy; Calibr: Research Funding; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Celgene/ Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Teclistamab is a BCMA-CD3 bispecific antibody approved for use in relapsed/refractory multiple myeloma. We will present two cases of patients with plasmablastic lymphoma treated with teclistamab with complete responses.