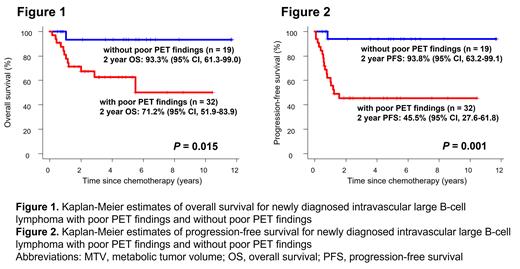
[Introduction] Treatment outcomes of intravascular large B-cell lymphoma (IVLBCL), a rare subtype of extra-nodal diffuse large B-cell lymphoma (DLBCL), have dramatically improved in recent years. However, prognostic factors for IVLBCL are largely unknown. Several studies have explored the potential of the baseline 18F-fluorodeoxyglucose (FDG) PET/CT radiomics features to identify patients with poor prognosis. For example, metabolic tumor volume (MTV) reflects the FDG-avid tumor burden, and the maximum distance between the largest lesion and any other lesion quantifies the dissemination of the tumor, and both factors are inversely related to overall survival (OS) and progression-survival (PFS) in DLBCL. In this study, we explored prognostic impact of FDG PET/CT radiomics features in newly diagnosed IVLBCL. [Methods] We retrospectively evaluated patients with histopathologically proven newly diagnosed IVLBCL according to the 2008 World Health Organization criteria between January 2010 through February 2022 from hospitals participating in the North Japan Hematology Study Group (NJHSG). Quantitative analysis of all FDG PET/CT was performed using Metavol (Hokkaido University, Sapporo, Japan), with exclusion of physiological accumulation. MTV was defined as the volume of lymphoma lesions visualized on PET/CT scans with standardized uptake value (SUV) peaks greater than or equal to hepatic SUV peak. To quantify the dissemination of the tumor, we calculated the number of the body areas (head-neck, chest, abdomen, upper-extremities, lower-extremities) having BM lesions with a higher SUV peak than liver SUV peak. Patients with BM lesions spread across 4-5 areas were defined as cases having widely disseminated lesions (WDL). Progression-free survival (PFS) was defined as the time from the start of treatment to the first of progression, relapse, or death from any cause. Patients undergoing upfront autologous stem-cell transplantation (auto-SCT) were censored at the time of transplantation. [Results] We identified fifty-two IVLBCL patients (age 30-89 years, median 65 years; 57.7% male) who performed FDG PET/CT at diagnosis. Twenty-eight patients (54.9%) had poor performance status (ECOG PS ≥2), and 49 patients (94.2%) showed stage III-IV. Forty-nine patients (94.2%) had 2 or more extranodal involvement. Elevated serum LDH level was observed in 49 cases (94.2%) (median, 623.5 IU/L; range, 147-4907 IU/L). The median serum levels of soluble IL-2 receptor (sIL-2R), ferritin, and C-reactive protein (CRP) were 4496.5 IU/mL (range, 321-19900 IU/mL), 655.15 ng/mL (range, 50-6657 ng/mL), and 6.905 mg/dL (range, 0.11-25.86 mg/dL), respectively. The median MTV was 415.527 cm 3 (range, 0-3000 cm 3). ROC analysis revealed that the cutoff value of the MTV > 388 cm 3 could predict progression events within 2 years. Area under curve was 0.71 (95% CI: 0.564-0.857). Twenty-nine patients (55.8%) were classified as having MTV > 388 cm 3 (high MTV). Twenty-three patients (44.2%) had WDL. Fifty-one (98.1%) patients received chemotherapy, all of which was rituximab-containing regimen. Three patients (5.8%) received upfront auto-SCT. Median follow-up duration was 48.8 months (range, 5.8-140.5 months) in survivors. Age, LDH level, number of extranodal lesions, clinical stage, and PS did not affect OS and PFS. Although the high MTV and WDL did not significantly affect OS ( P = 0.095, P = 0.173, respectively; Log-rank), these findings did correlate significantly with poor PFS ( P = 0.011, P = 0.042, respectively; Log-rank). Thus, we defined a high MTV or a WDL as poor PET findings. Thirty-two patients had poor PET findings. Importantly, patients with poor PET findings showed significantly poor OS and PFS compared to patients without (2-year OS: 71.2% vs. 93.3%, P = 0.015, 2-year PFS: 45.5% vs. 93.8%, P = 0.001; Log-rank, Fig. 1, 2). [Conclusion] This is the largest study to assess prognostic impact of FDG PET/CT radiomics features on newly diagnosed IVLBCL. Poor PET findings were significantly correlated with worse OS and PFS.
Disclosures
Goto:SymBio: Research Funding; Bristol-Myers Squibb: Research Funding; Novartis: Honoraria; Chugai: Honoraria; Kyowa Kirin: Honoraria, Research Funding. Horiguchi:Janssen pharmaceutical K.K: Honoraria; Chugai pharmaceutical: Honoraria; Otsuka Pharmaceutical: Research Funding; Abbvie pharmaceutical: Honoraria; Kyowa Kirin: Honoraria. Nakagawa:Mundipharma: Honoraria; Astrazeneca: Honoraria; Meiji Seika pharma: Honoraria; AbbVie Inc.: Research Funding; Takeda Pharmaceutical Company Limited: Honoraria, Research Funding. Hashimoto:Chugai Pharmaceutical: Honoraria; LUCA Science: Patents & Royalties; Kyowa-Kirin: Honoraria; Janssen Pharma: Honoraria; Ono Pharma: Honoraria; Daiichi Sankyo Inc: Honoraria; Astellas Pharma: Honoraria. Teshima:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Meiji Seika Pharma: Membership on an entity's Board of Directors or advisory committees; DAIICHI SANKYO: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Roche Diagnostics: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck Sharp & Dohme: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; LUCA Science: Research Funding; Otsuka: Research Funding; Priothera SAS: Research Funding; Chugai: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Fuji Pharma: Research Funding; NIPPON SHINYAKU: Honoraria, Research Funding; Asahi Kasei Pharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eisai: Research Funding; SHIONOGI: Research Funding; Sumitomo Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ONO: Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.