Duration of previous treatment, duration and depth of molecular response, previous resistance, BCR::ABL1 transcript, and lymphocyte subtypes are known predictive factors of molecular recurrence after tyrosine kinase inhibitors (TKI) discontinuation in chronic myeloid leukemia (CML) patients (pts). Few studies explored the kinetics of BCR::ABL1 during a dose de-escalation phase before discontinuation in predicting molecular relapse.
This study aimed to evaluate the kinetics of BCR::ABL1 transcripts during the de-escalation phase of the DES-CML trial and to correlate with molecular relapse after TKI discontinuation.
Methods: This prospective, multicenter, single-arm, phase 2 study included CML pts treated DES-CML Study. Inclusion criteria: Adult CML pts treated with TKI for a minimum of 3 years that achieved sustained deep molecular response (DMR), defined by a minimum duration of MR4.5 for two years, confirmed by four real-time quantitative reverse-transcription polymerase chain reaction (qPCR) tests with six months interval. Exclusion criteria: pts with atypical BCR::ABL1 transcripts, previous or current advanced stage CML, previous resistance to TKI or ABL mutations. The TKI dose was reduced by 50% for six months before discontinuation (de-escalation phase). Molecular monitoring was performed by qRT-PCR, using ABL as the control gene. The results were reported using the International Scale. BCR::ABL1 transcripts were evaluated monthly during the de-escalation phase. After discontinuation, assessments were performed monthly for six months, every two months at months 7-24, and then every three months. Therapy was restarted at the same dose before dose reduction if loss of MMR (qPCR<0.1%). Statistical analysis was performed by using SPSS software. Rates of MRFS were calculated by Kaplan-Meier analysis, and groups were compared using a log-rank test. P values of <.05 were considered statistically significant. This trial is registered at ReBEC (RBR-6f5xbq; UTN code: U1111-1252-7312) and was approved by the ethics committees of the participating centers. All pts signed informed consent.
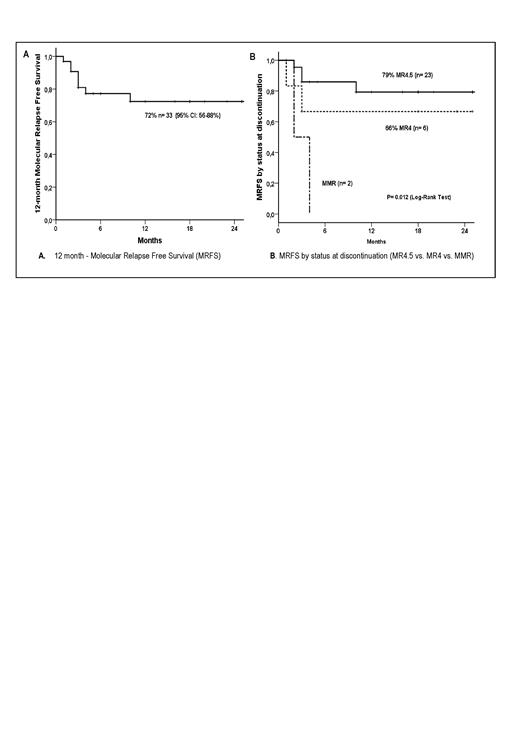
Results: This study is ongoing. Forty-one pts were enrolled from September 2020 to July 2023, with a median age of 61 years (23-85), 60% men, 33% Sokal low risk, 50% intermediate, 17% high; 78% with b3a2 BCR::ABL1 transcript type; 85% using imatinib, 7.5% dasatinib, 5% nilotinib, and 2.5% bosutinib. For 3 pts, it was the second attempt at stopping TKI. The median duration of TKI treatment until discontinuation was 11 years (3-20). The cutoff date was July 2023, with a median follow-up of six months (1-26) after TKI discontinuation. One patient had a screen failure; another was enrolled but has not started the de-escalation phase yet. Five patients are in the de-escalation phase, and 25 are in the discontinuation phase. Two patients did not complete the de-escalation phase due to a lack of medication and went to the discontinuation phase. One patient died from causes unrelated to CML in the discontinuation phase and was censored in the MRFS analysis. 15/33 (45%) patients lost MR4.5 at any time in the de-escalation phase, and 4/15 (31%) lost MMR after discontinuation. Three of the 18 patients who did not lose MR4.5 during de-escalation relapsed (16%, P=0.05). The two patients who did not undergo de-escalation were censored in this analysis. Nine pts lost MMR: one during de-escalation and 8/39 (20%) after discontinuation. The median time between discontinuation and loss of MMR was two months (0-10). The median time to MMR recovery was 1.5 months (1-5). The overall MRFS was 72% (95% CI: 56-88%)(Figure A) and 81% in the first-attempt discontinuation group. All pts in the second attempt relapsed (3 patients) (P<0.0001). There was no difference in MRFS by gender, BCR::ABL1 transcript type, Sokal, and ELTS. Molecular response status at discontinuation: 23 (74%) had MR4.5, 6 (19.5%) MR4.0, and 2 (6.5%) MMR. Patients with MR4.5 at discontinuation had a higher MRFS than those in MR4.0 and MMR (78% vs. 62% vs. 0%, respectively, P=0.012)(Figure B). No patient lost hematologic response or had disease progression.
Conclusions: Loss of MR4.5 during the de-escalation phase or molecular status not in MR4.5 before discontinuation was predictive of subsequent loss of MMR. Treatment-free remission was not successful in the second attempt of discontinuation.
Disclosures
Oliveira:Janssen: Speakers Bureau. Palma:Pfizer: Speakers Bureau. Figueiredo-Pontes:Novartis: Speakers Bureau; Pfeizer: Speakers Bureau. Pagnano:Pintpharma: Speakers Bureau; EMS: Research Funding, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.