INTRODUCTION
The advent of tyrosine kinase inhibitors (TKIs) has shifted the treatment paradigm of adult CML, limiting SCT to a last-resort option for TKI-ineligible or resistant cases. However, determination of the optimal dosing and duration, potential long-term side effects, and the viability of therapy discontinuation remain under-explored aspects of TKIs in pediatric CML. Emerging data supports administering novel TKIs as initial or second-line treatments in pediatric CML amid variable results. This review discusses the RCTs conducted on pediatric patients with CML who received TKIs, aiding in better understanding of the effectiveness and safety of TKIs in the pediatric CML population.
METHODS
We searched Medline, OVID, and Web of Science for all RCTs reporting the efficacy and safety of TKI in pediatric CML (< 18 years). We compiled the individual efficacy and safety data into distinct tables, emphasizing demographics, treatment modalities, and outcomes pertaining to both the safety and efficacy of TKI.
RESULTS
This review analyzed 17 Randomized Controlled Trials (RCTs) with 887 pediatric CML patients. Median age was 11.3 years (range 6.5-14 years). Efficacy studies had 718 patients, safety studies had 851.. The median white blood cell count was 234 x10 9/uL [range 19 to 378; reported in 9 RCTs], median hemoglobin level was 9 g/dL [range 5.6 to 10.8; from 7 RCTs], and the median platelet count was 431.5 x10 9/µL, [range 31 to 594; from 6 RCTs].
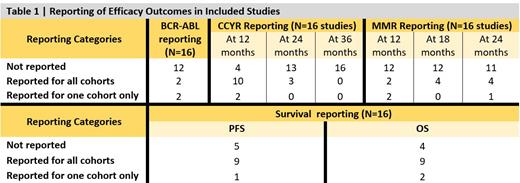
TKIs were first-line treatment in 65% of the studies, Imatinib being the commonest (61% of studies). Dasatinib and Nilotinib were used in 17% and 11% of studies, respectively, while 6% received a combination of imatinib and chemotherapy. Efficacy reporting in the RCTs was a major concern that challenged the accumulation of results (Table 1). BCR-ABL response of <10% ranged from 60 % to 78%, and the complete cytogenetic response (CCYR) at 24 months ranged from 62% to 94%. Progression free survival (PFS) ranged from 56.8% to 100%; however, the timepoint for PFS analysis was not standardized among studies (36 to 48 months).
Safety data from 16 studies (851 patients) included anemia (n=228), thrombocytopenia (n=161), neutropenia (n=257), hepatotoxicity (n=98), and cutaneous side-effects (n=156). Cardiovascular complications with second generation TKIs included QTc prolongation with Nilotinib (n= 11) and heart failure with Dasatinib (n=4). Nausea/vomiting were reported in 119 patients on imatinib, vs. 64 and 37 patients on Dasatinib and Nilotinib, respectively. Diarrhea occurred in 40 patients on Imatinib ,and 20 and 2 patients in Dasatinib and Nilotinib cohorts, respectively. Other AEs included impaired bone growth (70 on Imatinib and 10 on Dasatinib), headache (44 on Imatinib, 38 on Nilotinib and 13 in Dasatinib), musculoskeletal pain (161 on Imatinib, 31 on Dasatinib and 8 on Nilotinib). Treatment AEs leading to drug discontinuation were reported in 22 patients (6 studies), however the exact AE leading to drug discontinuation were not reported.
CONCLUSION
There remains a limited experience in treating pediatric CML with TKIs. Hence, evidence from prospective clinical trials and real-life clinical practice are required to establish appropriate guidelines for the standard therapeutic management in this population. Imatinib has the most extensice efficacy and toxicity data in pediatric patients. The Safety profile of TKIs was consistent with the known safety profile in adults. With the availability of three TKIs as first line options, multiple factors should be considered when selecting first line TKI, including drug formulation, administration, comorbidities, and financial issues. Careful monitoring of adverse events, especially in growing children should be considered in long term follow-up clinical trials.
Disclosures
No relevant conflicts of interest to declare.