Introduction: Nilotinib (NIL) is a potent second-generation tyrosine kinase inhibitor (TKI) approved for the treatment of newly diagnosed chronic myeloid leukemia (CML) patients (pts) in chronic phase, but reported to increase long-term toxicity such as cardiovascular (CV) risks. The ENESTfreedom study (Leukemia 2021) has reported 55% of treatment-free remission (TFR) rate after front line NIL discontinuation with a median 43.5 months of NIL treatment duration. Question still remains how to improve TFR rate after NIL frontline therapy. A potential solution is dose de-escalation (DESC) after MR4 achievement which could reduce the CV risk while extending MR4 duration prior to TFR attempt, thus increasing the chance of successful TFR attempt.
DESTINY trial (Lancet Haematology 2019) had attempted TKI dose DESC to half of the standard dose for 12 months before stopping TKI therapy, reporting only 3 pts (2.4%) lost molecular response after TKI dose DESC in 125 pts who achieved molecular response with 4 log or deeper (MR4). However, 85% of this cohort is mainly treated with imatinib, limiting applying this experience in NIL treated pts. Accordingly, the present study attempted to evaluate the efficacy of NIL dose DESC following MR4 achievement based on real-world experience.
Patients & Method: We have retrospectively analyzed the outcome of adult pts with CML in chronic phase treated with NIL frontline therapy and achieved sustained MR4 ( BCR::ABL1 transcript ≤0.01%IS). From the pts treated with NIL frontline therapy, we identified the cases attempted NIL de-scalation after achievement of MR4. The molecular response after NIL de-escalation was closely monitored every 3 months using BCR::ABL1 qPCR. Molecular recurrence was defined as an increase in BCR::ABL1 transcript levels above MR4 on at least two consecutive occasions or a single increase in BCR::ABL1 transcript level above MR3 ( BCR::ABL1 transcript ≤0.1%IS). At any time, pts who lost MR3 had returned Nilotinib dose to 300 mg BID. Primary endpoint of the study was event-free survival (EFS) following NIL de-escalation, which was defined from NIL DESC starts to molecular recurrence or death from any cause.
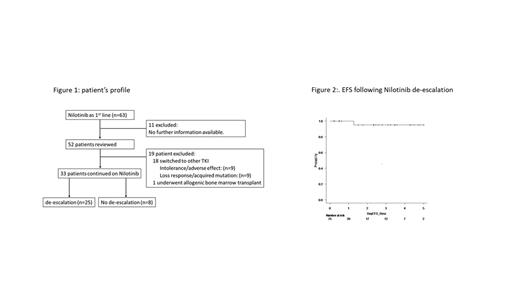
Results: We have identified 52 pts who started NIL as first line therapy for CML-CP under clinical practice. With median follow-up duration of 9 years (range:2-12 years), 33 patient (64%) remains on NIL at the last follow-up visit.
In 33 pts on NIL treatment, with a median follow up duration of 8 years (range;2-12), 25 (76%) had attempted NIL DESC at median of 6 years (range; 1-10 years) after frontline NIL therapy start, of whom the median time from NIL start to MR4 achievement was 12 months (range: 5-50 months). The median time from first achievement of MR4 to NIL DESC starts was 50 months (range: 3-105 months), while the median time from NIL start to NIL DESC was 77 months (range: 13-121 months).
At the latest follow up, only one patient experienced molecular recurrence at 3 months after DESC but regained MR3 in 9 months after re-escalation of NIL to 300mg BID. One patient died of cardiac arrest at 80 months after NIL DESC with other comorbidities of coronary artery disease, hypertension and diabetes. One patient discontinued NIL at 51 months post DESC and has sustained MR4 till last follow up. In the DESC group, the EFS at 3 years, 95% (69.5-99.3%). There is another case developed myocardial infarction while on DESC dose of NIL, who switched the treatment to Asciminib.
Conclusion: Achieving early and sustained MR4 with frontline NIL treatment facilitates DESC approach without compromising molecular response and might promote the TFR rate by extending MR4 duration. This DESC strategy can bridge CML pts on NIL frontline therapy prior to NIL discontinuation for TFR attempt.
Disclosures
Kim:Novartis: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Paladin: Consultancy, Honoraria, Research Funding; Merk: Consultancy; Sanofi: Consultancy, Honoraria.