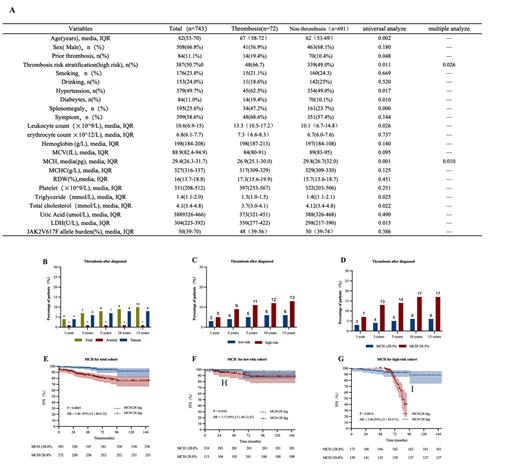
Thrombosis was a common complication and the main cause of
mobility of polycythemia vera (PV) with a lifetime incidence of 22%. Thrombosis risk was correlated with age, prior thrombosis, hypertension, red blood cell distribution width (RDW), leukocytosis, and JAK2V617F allele burden in previous studies, but only age≥65 years and prior thrombosis were included in thrombosis risk stratification. Finding independent thrombosis factors is significant to depict the landscape of PV and decrease the probability of thrombosis. Here, we aimed to find potential predictors to optimize the thrombocytosis risk stratification.
Retrospectively reviewed 763 patients diagnosed with PV according to the 2016 WHO criteria from 17 hematology center in China from Jan 1, 2010, to July 1, 2023. Analyzed clinical characteristics and genetic features of thrombosis risk and compared the potential predictor in different risk stratification.
The patients' clinical features are in table A.A total of 508(66.8%) subjects were male, the median age was 62 years (IQR, 53-70 years). The media follow-up was 45 months (IQR, 25-77 months). 84 (11.1%) subjects had thrombotic event pre-diagnosis, 25 (3.3%) of which were venous and 59 (7.9%) were arterial. 72 (9.6%) subjects had thrombotic events after diagnosis, 10 (1.3%) of which were venous and 62 (8.3%) were arterial (Figure B). Univariable analysis found thrombosis was associated with the thrombosis risk stratification (P=0.011), hypertension (P=0.017), diabetes (P=0.010), leukocytosis (P=0.026), mean corpuscular hemoglobin (MCH) (P=0.001), triglyceride (P=0.025), cholesterol (P=0.022), and splenomegaly (P=0.000). Thrombosis risk stratification (HR=1.74 [1.00, 3.02], P=0.026) and MCH (HR=0.92, [0.86, 0.98], P=0.010) were independently risk factors in multivariable variable analysis. We divided by MCH with cut-off point of 28.5pg, patients with MCH<28.5pg were associated with worse thrombosis-free survival (TFS) (Figure E). We further analyzed the effect of MCH in different thrombosis risk stratification. Results showed MCH <28.5pg was also meaningful in both low (P=0.0164) and high risk groups (P=0.0014) (Figure F, G). High risk stratification and MCH<28.5pg showed higher probability of thrombosis during the follow-up period (Figure C, D).
In our study, potential risk factors from other studies such as JAK2V617F allele burden, hyperlipidemia, hypertension, leukocytosis, and RDW were not significant independent predictors of thrombosis. However, we found MHC<28.5pg was correlated with worse TFS both in low thrombosis risk stratification group and high thrombosis risk stratification group. Our study suggested that MCH< 28.8pg was an independent predictor and can increase the accuracy of predicting thrombosis.
Acknowledgement: This research was funded by the Key R&D Program of Zhejiang, No. 2022C03137; Public Technology Application Research Program of Zhejiang, China, No. LGF21H080003; Zhejiang Medical Association Clinical Medical Research special fund project, No. 2022ZYC-D09.
Correspondence to: Dr Jian Huang, Department of Hematology, The First Affiliated Hospital of Zhejiang University School of Medicine. househuang@zju.edu.cn
Disclosures
No relevant conflicts of interest to declare.