Introduction Belantamab mafodotin (belamaf; GSK2857916) is a B-cell maturation antigen-binding antibody-drug conjugate approved as a monotherapy in pretreated patients (pts) with relapsed/refractory multiple myeloma. Ocular adverse events (OAEs), manifesting as visual acuity changes, ocular symptoms, and corneal findings, are common with belamaf and the main reason for dose modifications (dose reduction, dose holds) or treatment discontinuation. In this descriptive analysis, we evaluate the OAEs and their impact on daily functioning in transplant ineligible (TI) pts with newly diagnosed multiple myeloma (NDMM), treated with an extended dosing schedule of belamaf in combination with lenalidomide and dexamethasone (Rd) in the phase 1/2 BelaRd study.
Methods BelaRd (NCT04808037) is an open-label, phase 1/2 study conducted in Greece, aiming to enroll 66 TI NDMM pts. The study is comprised of 2 Parts: Part 1 evaluates the safety/clinical activity of three different belamaf doses (2.5/1.9/1.4 mg/kg) plus Rd and establishes the recommended phase 2 dose (RP2D). Part 2 further investigates the safety/efficacy of RP2D in two Groups and evaluates two different sets of guidelines for the management of OAEs. In Part 1, belamaf is initially administered q8w and, depending on toxicity, dosing may be adjusted to q12w. Ocular assessments include best corrected visual acuity (BCVA), using Snellen chart and manifest refraction, and corneal exam using slit lamp microscopy. Ocular symptoms are classified by Common Terminology Criteria for Adverse Events v5.0, while dry eye disease severity and vision-related functioning is assessed with the patient-reported questionnaire Ocular Surface Disease Index (OSDI). In Part 1 of the study, severity of corneal events is assessed with the Keratopathy Visual Acuity scale. This descriptive analysis presents the OAEs in all Part 1 pts over an extensive follow-up period (data cut-off: 05 June 2023).
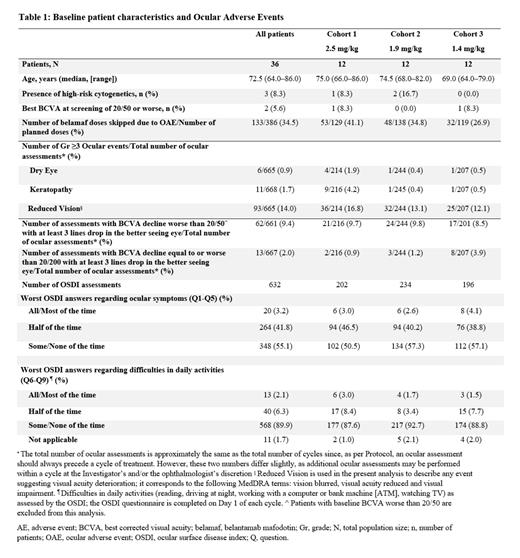
Results Thirty-six pts were included in this analysis, who were followed up for a median of 20.3 months (range 3.2-26.8). At baseline, all pts had ocular comorbidities. Among 216/244/201 BCVA assessments in cohorts 2.5/1.9/1.4 mg/kg, a meaningful BCVA decline (BCVA <20/50) with at least 3 lines drop in the better seeing eye, was observed in 21 (10%)/24 (10%)/17 (8%) of the assessments, while BCVA ≤20/200 with at least 3 lines drop in the better seeing eye was noted in only 2 (1%)/3 (1%)/8 (4%), with a median time to resolution of 1 month. The most frequently reported ≥Gr 3 ocular symptom was decreased vision, observed in 11 (5%)/ 8 (3%)/ 7 (3%) of the assessments, while ≥Gr3 keratopathy was noted in < 2% of assessments across all cohorts. Regarding OSDI, from 202/234/196 responses received, the number of “all/most” of the time worst responses in the ocular symptoms category were 6 (3%)/6 (3%)/8 (4%), while the respective proportions in the Activities of Daily Living category (ADL) were 6 (3%)/4 (2%)/3 (2%). In terms of missed doses due to OAEs, among 129/138/119 planned belamaf infusions, the number of skipped doses were 53 (41%)/48 (35%)/32 (27%). The overall response rate was 100%, no disease progression was observed over a median follow-up of 20.3 months, and 30 (83%) patients achieved at least VGPR while 19 (53%) achieved at least CR, with a median time to first response of 1 month.
Conclusions Over a prolonged follow-up period, the belamaf-Rd triplet, with the extended dosing schedule for belamaf, had a minimal impact in vision-related functioning, as the ‘all/most’ of the time worst answers in the ADL category of OSDI was ≤3% across all cohorts. Furthermore, the frequency of clinically relevant impairment in vision was low, as a meaningful BCVA decline was observed in <10% of assessments, with a rapid time to resolution. Finally, the treatment combination induced rapid, deep and durable responses across all dose levels, with no PD observed. In conclusion, this novel extended belamaf schedule nearly eradicates the risk for clinically relevant ocular toxicity and impact on ADL, without any compromise in clinical activity, suggesting it may be a valid option in the upfront treatment of this frail, elderly and difficult-to-treat pt population.
Disclosures
Terpos:GSK: Honoraria, Research Funding; Amgen: Honoraria, Other: Travel Expenses, Research Funding; BMS: Honoraria; ASTRA/Zeneca: Honoraria, Other: Travel Expenses; EUSA Pharma: Honoraria, Other: Travel expenses; Janssen: Honoraria, Research Funding; Menarini/Stemline: Honoraria; Pfizer: Honoraria; Sanofi: Honoraria, Other: Travel expenses, Research Funding; Takeda: Honoraria, Other: Travel expenses, Research Funding. Gavriatopoulou:Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; X4 Pharmaceuticals: Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene/Genesis: Honoraria; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees. Migkou:Glaxo Smith Klein: Honoraria; Integris Pharma: Honoraria; Janssen-Cilag: Honoraria. Gkolfinopoulos:Heads: Current Employment. Kastritis:Janssen: Honoraria, Research Funding; Sanofi: Honoraria; Pfizer: Honoraria, Research Funding; GSK: Honoraria, Research Funding. Dimopoulos:BeiGene Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini: Honoraria, Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees.