Background: Light-chain amyloidosis (AL) is a rare, multisystem disease caused by plasma cell dyscrasia (PCD) with a varied presentation, and significant morbidity and mortality. The current standard of care is anti-PCD treatment with a combination of cyclophosphamide-bortezomib-dexamethasone (CyBorD) often supplemented with daratumumab based on National Comprehensive Cancer Network guidelines. These regimens halt amyloid formation but do not treat existing amyloid deposits in organs. Delays in diagnosis and treatment initiation can result in poor survival, particularly in patients with cardiac involvement.
Aims: To elucidate the treatment patterns for patients with AL using US-based claims data.
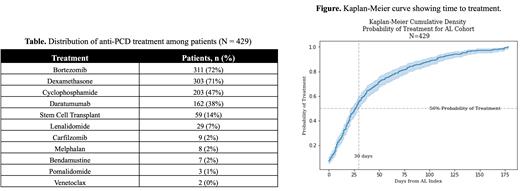
Methods: We conducted a retrospective cohort study of adults (age ≥18 years) with AL using IQVIA PharMetrics Plus data (1 January 2016, to 30 September 2022). Patients with ≥2 AL diagnosis codes (index is date of first diagnosis; ICD-10: E85.81), ≥24 months' continuous coverage pre-index, ≥6 months follow-up, and treated within 6 months of index date were included. Results are reported using descriptive statistics. Median time-to-treatment and cumulative probability of treatment were computed using the Kaplan-Meier method.
Results: A total of 429 patients (60% male, mean age 62 years) met inclusion criteria. Patients most often received bortezomib (72%). Other common anti-PCD treatments ( Table) included dexamethasone (71%), cyclophosphamide (47%) and daratumumab (38%). The median time to treatment ( Figure) was 27 days (95% CI: 22, 30) post-index. In this cohort, 239 (56%) patients received treatment within 30 days of the index date; treatment was initiated >30 days of index date for the remainder (190; 44%) of patients. Fifty-nine (14%) patients received autologous stem cell transplant (ASCT) within 6 months of index date of which 14 (3%) received no induction therapy prior to transplant. ASCT rates decreased slightly throughout the study period (16% in 2018 to 11% in 2022). Visits to the emergency room (ER) for any cause during the 6-month follow-up period were reported for 138 (32%) patients; 97 (23%) patients visited the ER for cardiovascular-related reasons. The odds of experiencing an all-cause ER visit over the follow-up period was 12% lower (crude, unadjusted odds ratio [OR] of 0.88; p = 0.549) for patients who treatment within 30 days of index date compared with those for whom treatment was initiated >30 days of index. The odds of experiencing a cardiovascular-related ER visit over the follow-up period was 5% higher (crude, unadjusted OR of 1.05; p = 0.823) for patients who treatment within 30 days of index date compared with those for whom treatment was initiated >30 days of index.
Conclusions: This study outlines treatment patterns within a US claims-based cohort of patients with AL. Few studies report time-to-treatment initiation in AL, and to our knowledge none have explored whether time-to-treatment initiation may impact likelihood of ER visits. The median time to treatment of 27 days is shorter than historic reports but still requires improvement. 1
We demonstrate that while almost half of patients in this study did not start therapy within 30 days of index date, they did not appear significantly more likely to have ER visits. This may be because those whose treatment was delayed had less severe disease and those treated more promptly had more severe disease. However, these results should be interpreted with caution as the analysis was not adjusted for confounding factors.
The majority of patients in this study were treated with bortezomib and dexamethasone, almost half with cyclophosphamide, and 38% with daratumumab. The uptake of ASCT in this cohort was substantially lower than that reported by US amyloidosis specialist centers. 2,3 Uptake of ASCT in this study was more in line with the ~20% of newly diagnosed patients in AL that are generally regarded as transplant eligible. ASCT uptake appeared to fall slightly to 11% of patients in 2021-2022, potentially with the January 2021 approval of daratumumab - although overall numbers were too small to make this conclusion. Further efforts are needed to understand the real-world uptake of ASCT in newly diagnosed AL with the increasing use of CyBorD with daratumumab.
References:
1. Catini J. et al. Blood. 2022;140 (Suppl 1):4330.
2. Muchtar E. et al. Blood. 2017;129:2111.
3. Staron A. et al. Blood Cancer J. 2021;11:139.
Disclosures
Lyons:Alexion, AstraZeneca Rare Disease: Current Employment. Thompson:Alexion: Current Employment. Lousada:Alexion, AstraZeneca Rare Disease: Consultancy. Maurer:Alexion, AstraZeneca Rare Disease: Consultancy. Catini:Alexion, AstraZeneca Rare Disease: Current Employment. Manwani:AstraZeneca: Current Employment.