Introduction
This project aimed to provide an overview of the treatment landscape for AL - Amyloidosis (AL-A) in Austria as documented in AIDA, the Austrian Interdisciplinary Amyloidosis Registry. By examining the existing body of knowledge surrounding treatments and demographics, we seeked to shed light on the potential benefits and challenges for new treatment modalities in the context of AL-A.
Methods
We identified 210 AL- pts. from AIDA (cut off May 15, 2023). Statistics were descriptive. Kaplan Meier was chosen for survival plots and analyses were carried out with Excel and SPSS.
Results
We present data from 210 AL-A pts. recruited from AIDA. This constitutes 45.95% of all pts. in AIDA, with the major parts of the residual population made up by wt-ATTR amyloidosis pts. (47.48%), with some rare other amyloidosis subtypes accounting for the rest.
There was a male pre-dominance in the cohort (62.38%) well in line with published data.
Pts. with AL-A were found to be much younger (median age 66a vs. 78a) compared to pts. suffering from wt-ATTR. Diagnosis of AL-A was made between 1997 and 2023, with the median year of first diagnosis being 2016, resulting in median follow up in the cohort of ~ 7 years.
The pattern of organ involvement by AL-A were quite typical with heart (78.6%), kidney (58.1%), GI-tract (23.3%) and PNS (21.4%) being the dominant manifestations. Advanced stages by either the classical Mayo staging, as well as the revised one were regrettably still by far more frequent at time of diagnosis as early disease stages (classical Mayo III in 65.8%, and revised MAYO III and IV in 27.8% and 42.3% of pts. respectively). This is mirrored in a high number of pts. with severe to moderate renal insufficiency (GFR < 30ml/min in 4.6% and between 30-60 ml/min in 42.2% of pts.) and/or moderate to severe cardiac involvement (NT-pro BNP > 5000 ng/l in 37.2%, and between 500 - 5000 ng/l in another 51.2% of pts.). Detailed data on comorbidities and toxicities will be added.
Treatments
Only 141/210 pts. ever received a documented 1 st line of treatment (LoT), of which only 35.5% received a 2 nd LoT, and even less a 3 rd LoT (20.6%), while more LoTs were applied only in a handful of pts. This horrendous attrition rate per LoT is far above, what has been described for Myeloma (20% / LoT). Time to next tretament was found to be median of 9.9 months. 54 pts. received a 1st LoT with the intent of pursuing an autologous stem cell transplantation (ASCT), but only 54% of these actually were transplanted. This compares inferior to Myeloma, although MM pts. are usually significantly older. The most frequently used therapeutic regimes were VCd-D (Andromeda) with or without Dara-maintenance. The widespread use of anti-CD38 based treatments in this sub-cohort is mirrored in a quite high rate of high-quality hematologic responses (CR+VGPR = 51.9%).
90 pts. received 1st LoTs not intending to lead to ASCT. Again, Daratumumab based regimes were the most used, but treatment outcomes were much inferior (hematologic CR+ VGPR = 18.9%) probably based on dosages, time on therapy, and not applied ASCT.
Only 40 pts. received conventional 2nd LoTs usually classical Myeloma therapies, with modest treatment results. Even hematologic high-quality responses (CR+VGPR = 25%) were rare not to mention organ responses.
Survival
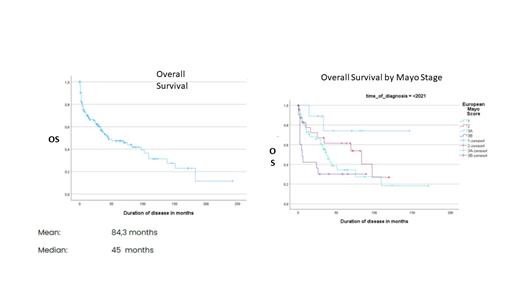
Median survival in the AIDA cohort was found to be 45 months. Broken down by Mayo stages, both classic and revised, was found to be quite unfavorable for advanced disease (14 months in revised Mayo stage IV or classical Mayo stage III).
Conclusions
Compared to other plasma cell dyscrasias overall outcomes in AL-A are still dismal, despite the introduction of recent effective anti-plasma cell therapeutics in the treatment algorithm. Disease modifying therapies are urgently needed and should be preferentially introduced in 1 st line, as only few pts. will receive 2 nd or later LoTs.
Furthermore, efforts to improve awareness for this dreadful disease are urgently needed to enable more therapies to be applied in less advanced disease settings.
Take home-messages:
1. Efforts needed to improve early diagnosis of AL-A when treatment options will not be hampered by progressive organ dysfunctions
2. Absolute necessity to explore disease modifying therapies going beyond stopping excess free light chain production
3. As only a limited number of pts. received multiple LoTs any new therapeutics should be integrated in effective 1 st LoTs preferentially.
Disclosures
Willenbacher:syndena GmbH, connect to cure: Current Employment; Alexion: Research Funding. Pichler:Amgen, Gilead, Janssen, Sanofi-Aventis, Takeda: Honoraria. Rittler:syndena GmbH, connect to cure: Current Employment. Weger:syndena GmbH, connect to cure: Current Employment.