Background and Significance:
Multiple myeloma (MM) evolves from the precursor state monoclonal gammopathy of undetermined significance (MGUS). According to previous reports, the incidence of MM and its precancerous state MGUS was lower in Asian populations than in Western populations. However, with the aging of the population, we found that the incidence of MM in China has been on the rise in recent years, and the medical burden has continued to increase. Therefore, early recognition of MM and its precursor condition is even more critical. Our previous research has confirmed that monoclonal gammopathy (M-protein) screening and close follow-up of MGUS can increase the early diagnosis rate of MM and improve the prognosis of MM patients[1].
M-protein screening in healthy people at high risk for MM is a rational strategy. The risk of MM in the first-degree relatives of myeloma patients is 2-4 times that of the general population, constituting a high-risk group for MM. Although the screening of M-protein in high-risk populations has been explored in the US (the PROMISE study), little was known about the incidence, clinical features, and prognosis of MGUS in high-risk groups in China. Here, we describe the first prospective screening project to assess the potential benefits of M-protein screening and the prevalence and outcomes of MGUS in first-degree relatives of patients with multiple myeloma in the China population (NCT05965115).
Study Design and Methods:
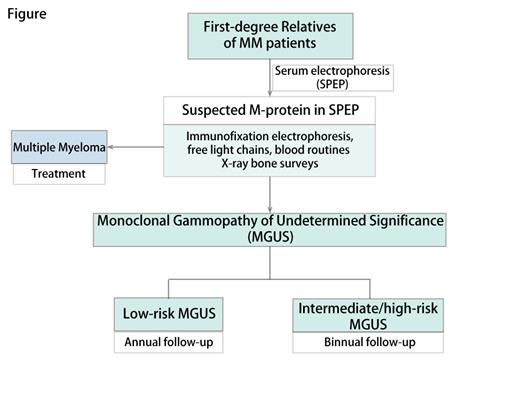
The CHAPERONE study is a prospective, multicenter screening study for MGUS in first-degree relatives of MM patients, including a risk-stratified follow-up strategy(Figure).
Clinical trial registry number: NCT05965115, actively recruiting.
This study aims to assess the clinical significance of screening for monoclonal gammopathy in first-degree relatives of patients with multiple myeloma in China. The primary objective is to determine the prevalence and incidence rate of MGUS, the time to disease progression (TTP), and its influencing factors in first-degree relatives of MM patients in China. The secondary and exploratory study objective is to explore the clinical/genomic features, survival, and quality of life of the screened MGUS cases.
The expected screening size is 10,000 healthy persons. To be eligible, participants must be over 18, with one or more first-degree relatives (including parents, children, and biological siblings) having multiple myeloma diagnosed by the International Myeloma Working Group (IMWG) Myeloma Diagnostic Criteria. Participants with a known diagnosis of plasma cell diseases, such as MGUS, smoldering myeloma (SMM), or MM, will be excluded.
All eligible participants will receive M-protein screening by serum electrophoresis. Individuals with detected M-protein will undergo further diagnostic tests, including peripheral blood tests (immunofixation electrophoresis, serum free light chain, immunoglobulin quantification, blood routine, liver and kidney function, and blood calcium level) and X-ray bone assays. Patients with suspecting MM features will undergo further bone marrow surveys to ascertain the diagnosis. Patients with MGUS will be carefully assessed and stratified by the risk of progression to MM according to the IMWG MGUS consensus. Low-risk MGUS will be followed-up annually, while intermediate/high-risk MGUS will be biannual. Participants who progress to MM will be offered early treatment.
Conclusions and Future Directions:
One path to making MM a preventable cancer is better understanding its precursor condition (MGUS). This trial is positioned to provide epidemiology, clinical, and potential mechanistic data on MGUS at a high-risk group in China population that has yet to be thoroughly studied.
Reference:
[1] Li J, Wang Y, Liu P. The impact on early diagnosis and survival outcome of M-protein screening-driven diagnostic approach to multiple myeloma in China: a cohort study[J]. J Cancer, 2019, 10(20): 4807-4813.
Disclosures
No relevant conflicts of interest to declare.