Background Patients with refractory immune thrombocytopenia (ITP) often suffer severe bleeding events and have poor responses to various treatments. Umbilical cord-derived mesenchymal stem cells (UC-MSCs) have been used to treat multiple autoimmune diseases due to their low immunogenicity and strong immunomodulatory potential. To explore the safety and efficacy of UC-MSCs in treating refractory ITP, we conducted this phase Ⅰ clinical trial.
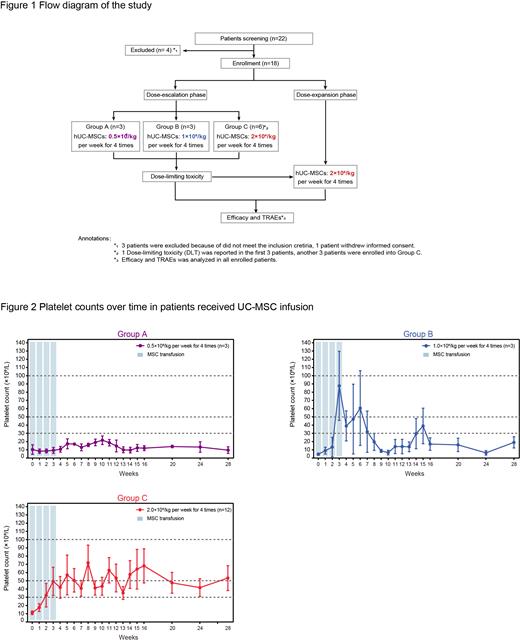
Methods Patients diagnosed with refractory ITP were screened for the study. The major inclusion criteria included age 18 to 60 years, ITP duration lasting for more than six months, platelet count < 30 × 10 9/L and concomitant bleeding manifestations at enrolment. The study consisted of two parts: a dose-escalation phase for exploring the safety and efficacy of three different dosage groups according to the traditional 3+3 protocol and a dose-expansion phase for further verifying the optimal UC-MSC dosage. UC-MSCs were administered at doses of 0.5×10 6 cells/kg, 1.0×10 6 cells/kg, and 2.0×10 6 cells/kg per week four times in the dose-escalation phase and 2.0×10 6 cells/kg per week four times in the dose-expansion phase. The adverse events, platelet counts, and bleeding symptoms were evaluated and recorded during the administration and follow-up period. The study protocol was approved by the Ethics Committees of the Institute of Hematology and Blood Diseases Hospital, and informed consent was obtained from each participant according to the Declaration of Helsinki (ClinicalTrials.gov ID: NCT04014166).
Results Between November 2019 and August 2022, 18 refractory ITP patients hospitalized in our centre were successfully screened and enrolled in the study. Twelve patients were enrolled in the dose-escalation phase (three additional patients were added in the 2.0×10 6 cells/kg group due to the dose-limiting toxicity), and six patients were included in the dose-expansion phase. Thirteen patients (13/18, 72.2%) had one or more TEAEs. SAEs occurred in four patients (4/18, 22.2%), including gastrointestinal haemorrhage (2/4), profuse menstruation (1/4), and acute myocardial infarction (1/4). There were 0.0% (0/3), 66.7% (2/3), and 50.0% (6/12) patients in the 0.5×10 6, 1.0×10 6 and 2.0×10 6 cells/kg cohorts who achieved a response after treatment, respectively. The overall response rate was 44.4% (8/18) in all patients who received UC-MSCs.
Conclusion UC-MSC infusion achieved a 44.4% overall response rate in patients with refractory ITP and mild adverse events.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
Mesenchymal stem cells have no clinically approved indications in ITP treatment. Current data show that more than 10 mesenchymal stem cell products have been approved for clinical research qualifications in the world, mainly for the immune regulation and injury of graft-versus-host disease (GVHD), Crohn's disease, myocardial infarction, osteoarthritis and other diseases repair.