CAR-T cell therapy has revolutionized the treatment of adult patients with relapsed/refractory (r/r) non-Hodgkin lymphomas (NHL) and multiple myeloma (MM). While lymphodepletion (LD) with fludarabine/cyclophosphamide (flu/cy) has been the standard, the fludarabine drug shortage has caused a shift toward the use of bendamustine as an alternative. This study expands upon previous investigations evaluating bendamustine as a LD agent prior to CART therapy.
We conducted a retrospective study of 48 patients with r/r NHL (marginal zone lymphoma (MZL), diffuse large B cell lymphoma (DLBCL), mantle cell lymphoma (MCL), and follicular lymphoma (FL)) and MM who received bendamustine LD prior to treatment with commercial CART products at the UPMC Shadyside Hospital between 07/2022-04/2023. Data were collected using chart review and analyzed by descriptive statistics. Responses for NHL and MM were assessed based on the Lugano 2014 criteria and the International Myeloma Working Group consensus criteria, respectively. Toxicities were evaluated based on the American Society for Transplantation and Cellular Therapy consensus grading and the Common Terminology Criteria for Adverse Events.
33 patients with NHL (24 with DLBCL, 2 with MZL, 2 with FL and 5 with MCL) and 15 with MM, were included with a mean age of 65.8 years. The median number of prior therapies was 2 [1 - 5] for NHL, and 5 [4 - 10] for MM. In the NHL cohort, 14/33 patients were treated in the second line. Among NHL patients, 5/33 patients received brexucabtagene autoleucel, 24/33 axicabtagene ciloleucel (axi-cel), and 4/33 lisocabtagene maraleucel (liso-cel). Among MM patients, 2/15 patients received idecabtagene vicleucel, and 13/15 ciltacabtagene autoleucel (cilta-cel). Grade (G) 1-2 CRS occurred in 23/33 of NHL and 14/15 of MM patients, without any reported G3 CRS. ICANS was reported in 9/33 (3 G3) of NHL and 7/15 (1 G3) of MM patients. The overall response rate (ORR) in MM patients was 12/15, with 6/12 achieving complete response (CR) or stringent CR (sCR) and 6/12 a very good partial response (VGPR), and only 1/12 progressing after a response. In the NHL cohort, we evaluated the ORR for every subtype. The ORR was 16/24 (11/16 CR and 5/16 PR) in DLBCL, 1/2 (CR) in MZL, 3/5 (all CR) in MCL, and 2/2 (all CR) in FL. Only 3 patients with DLBCL, 1 with MZL and 1 with MCL eventually progressed after a response. These response results are comparable with prior CART studies of flu/cy LD that have shown ORR range of 72-73% (CR 51-54%) for DLBCL ( Locke F, Lancet Oncol., 2019; Abramson JS, Lancet 2020), 91% (CR 60%) for FL ( Jacobsen CA, Lancel Oncol. 2022), and 98% (sCR 78%) for MM ( Berdeja JG, Lancet 2021).
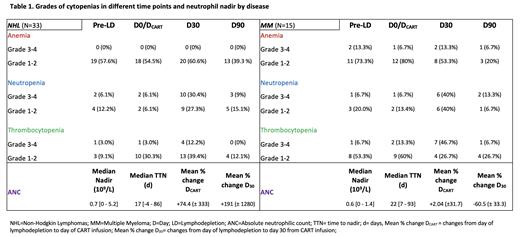
Next, we evaluated the change in hematologic parameters after LD. Absolute lymphocyte counts (ALC) showed mean decreases of 66.8% (± 28.4%) in NHL and 69.3% (± 16.4%) in MM from pre-LD to the day of CAR-T infusion (D CART), with median time-to-nadir (TTN) post-D CART of 1 [0-13] days in NHL and 1 [0-8] days in MM. We then compared the incidence of neutropenia in our cohort relative to historical controls treated with flu/cy LD. Neutropenia was seen in 80% of patients (G≥3, 68%) with axi-cel, 50% (G≥3, 50%) with liso-cel, and 100% (G≥3, 77%) with cilta-cel. This compares favorably to historical data with axi-cel, (86%; G≥3, 80%), liso-cel (63%; G≥3, 60%), and cilta-cel (96%; Gr≥3, 95%). Table 1 illustrates the grades of cytopenias and ANC nadir and TTN post-D CART in our study.
Finally, we investigated the mean change in inflammatory markers as a surrogate of CAR-T activation and expansion. CRP showed a median increase of 2.8-fold [0 - 66.5] in a median of 5 [0-55] days in NHL, and a median increase of 4.6-fold [0.22 - 74.9] in a median of 8 [2-15] days in MM. Ferritin had a median increase of 1.2-fold [0- 50] in median of 9 [0-171] days in NHL, and median increase of 3.8-fold [0.63 - 59.4] in median 10 [0-148] days in MM.
In conclusion, bendamustine is a successful LD agent prior to CAR-T infusion, yielding response rates comparable to historical controls in NHL and MM. Notably, our study reveals a favorable safety profile with a lower rate of high grade cytopenias than was seen in the pivotal trials using flu/cy LD. These findings are reassuring regarding the use of bendamustine as an alternative LD agent in the face of the ongoing fludarabine drug shortage. Further studies assessing CART copy numbers and persistence of CART are needed.
Disclosures
Shlomchik:Orca Bio: Consultancy, Current holder of stock options in a privately-held company; BlueSphere Bio: Current Employment, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding. Dorritie:BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Curio and Dava Oncology: Honoraria; Genentech: Research Funding; Kite, a Gilead Company: Research Funding; Janssen: Research Funding; Hoffman-LaRoche: Research Funding; Genmab: Research Funding.