Introduction
The type of conditioning regimen has a great impact on the outcome of patients who undergo allogeneic HSCT since graft versus host disease (GVHD), infections, regimen related organ and tissue toxicities are among the most important causes of post-transplant mortality. Despite the regimen related toxicity profile of busulfan, supported by information from the results of clinical trials and real-world data, it is still commonly used worldwide. Treosulfan has advantages in terms of dose of administration, lower incidence of sinusoidal obstruction syndrome and lower neurotoxicity. We aimed to retrospectively investigate outcomes and compare results of patients who underwent matched and haploidentical allogeneic HSCT with treosulfan based or busulfan based reduced intensity conditioning, non-myeloablative conditioning and myeloablative conditioning regimens in our institution.
Methods
From September 2016 to November 2022, treosulfan was administered to 94 patients while 85 patients received busulfan. We analyzed outcomes as regimen related-toxicity, chronic and acute GVHD, overall survival, non-relapse mortality, relapse related mortality and fungal infection. The myeloablative dose of busulfan was 12.8 mg/kg while it was 9.6 mg/kg for the non myeloablative regimen which was combined with fludarabine. The myeloablative dose of treosulfan was 42 mg/m2 while a dose range of 30-36 mg/m2 was administered for the reduced intensity conditioning.
Results
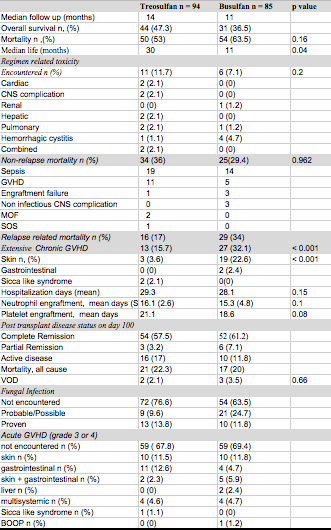
The median follow up was 14 months for the treosulfan group while it was 11 months for the busulfan group (p = 0.16). Regimen related toxicity, which was monitored during the hospital stay and 3 months after discharge were 11.7% and 7.1% for treosulfan and busulfan respectively and were similar between two groups. Extensive chronic GVHD was encountered in 15.7% of the patients in the treosulfan group compared to 32.1% in the busulfan group (p < 0.001). Similarly, chronic skin GVHD was observed in 3.6% of the patients who received treosulfan while it was seen in 22.6% of the busulfan group (p < 0.001). Acute GVHD (grade 3 or higher) was encountered in 32.2% of the patients within the treosulfan group while it was encountered in 31.6% of the patients in the busulfan group. Overall survival was 47.5% and 36.5 % in the treosulfan group and busulfan groups respectively. The relapse-related mortality was 17 % in the treosulfan group while it was 34% in the busulfan group. Fungal infection was observed in 23.4% and 36.5% of the patients in treosulfan and busulfan groups respectively.
Conclusion
Treosulfan, with a lower chronic GVHD incidence and similar regimen related toxicity profile appears to be a safe alternative to busulfan and prospective randomized controlled trials are needed to confirm the results in our study.
Keywords: Busulfan; treosulfan; conditioning regimen; stem cell transplantation
Disclosures
No relevant conflicts of interest to declare.