Objective: Acute graft-versus-host disease (aGVHD) is a common complication following allogeneic hematopoietic stem cell transplantation. Currently, the reported incidence of moderate to severe aGVHD in domestic cases is 13%-47%, while the incidence of grade III/IV aGVHD after haploidentical transplantation ranges from 7.9% to 13.8%. Severe aGVHD is one of the leading causes of early post-transplant mortality. This study aims to analyze whether the administration of Brentuximab vedotin after transplantation can reduce the incidence of aGVHD without increasing disease relapse rates and viral infections.
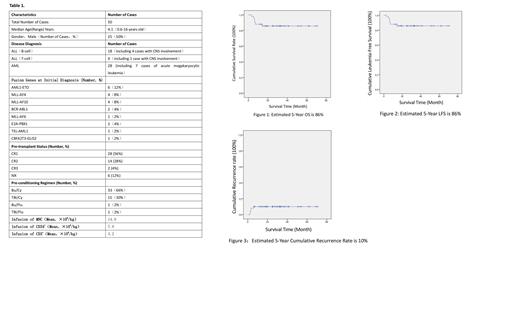
Methods: A retrospective analysis was conducted on 50 pediatric leukemia patients who underwent haploidentical hematopoietic stem cell transplantation (HSCT) at our hospital between June 2017 and July 2022. These patients received prophylactic treatment with Brentuximab vedotin (20mg) on day +1 and day +4 post-transplantation to prevent aGVHD. The median age of the patients was 4.1 years (range: 0.6-16.0 years), with a gender ratio of 25:25 (male:female). At the time of diagnosis, the median white blood cell (WBC) count was 19.9×10 9/L (range: 2.0-863×10 9/L) for 41 patients, while the WBC count at initial diagnosis was unknown for 9 patients. Among the cases, 22 were acute lymphoblastic leukemia (ALL), and 28 were acute myeloid leukemia (AML). Before transplantation, 28 patients were in CR1, 14 in CR2, 2 in CR3, and 6 in NR. All patients received myeloablative conditioning regimens, with 33 receiving Bu/Cy, 15 receiving TBI/Cy, 1 receiving Bu/Flu, and 1 receiving TBI/Flu. The median follow-up time was 29.1 months (range: 3.1-70.1 months). Detailed patient data are presented in Table 1.
Results: All 50 patients achieved 100% engraftment of white blood cells, with a median time of 15 days (range: +10 to +21 days). Platelet engraftment occurred in a median time of 9 days (range: +5 to +78 days), except for one patient who did not achieve platelet engraftment. This particular patient was diagnosed with AML and experienced hematologic relapse and extramedullary relapse before transplantation. The patient died due to disease relapse 90 days after transplantation.The incidence of aGVHD after transplantation was 44%, with 28% experiencing grade I-II and 16% experiencing grade III-IV aGVHD. The incidence of CMV viremia was 40%, with a median time to diagnosis of 36 days (range: +19 to +84 days), while the incidence of EBV viremia was 8%, with a median time to diagnosis of 47 days (range: +14 to +87 days). During the follow-up period, five patients experienced disease relapse, with a median time to relapse of 92 days after transplantation (range: +67 to +181 days). Among the relapsed patients, 2 had ALL (both in CR2), and 3 had acute myeloid leukemia (all in NR). Seven patients died during the follow-up, including 4 with AML and 3 with ALL. The causes of death were disease relapse in 3 cases, infection in 2 cases, TMA in 1 case, and cGVHD in 1 case. The estimated 5-year overall survival (OS) and leukemia-free survival (LFS) rates were 86%, and the 5-year cumulative relapse rate was 10% (see Figures 1, 2, and 3). In comparison, a control group of 44 pediatric acute T-cell lymphoblastic leukemia patients who underwent haploidentical transplantation without prophylactic Brentuximab vedotin treatment between January 5, 2021, and June 30, 2022, were analyzed. In the control group, the incidence of aGVHD was 56.8% (P=0.007), with 36.4% experiencing grade I-II and 20.4% experiencing grade III-IV aGVHD. The incidence of CMV viremia was 68.2% (P=0.006), and the incidence of EBV viremia was 29.5% (P=0.007). The relapse rate was 15.9%, and the mortality rate was 27.3%.
Conclusion: For pediatric acute leukemia haploidentical HSCT, the administration of Brentuximab vedotin (20mg) on days +1 and +4 after transplantation, in addition to conventional aGVHD prophylaxis (CsA+MMF+sMTX), significantly reduced the overall incidence of aGVHD and grade I-II aGVHD. Administering Brentuximab vedotin on days +1 and +4 did not increase the rates of CMV or EBV infection nor did it increase the risk of disease relapse.
Disclosures
No relevant conflicts of interest to declare.