Background: despite rapid development of new highly efficient targeted and cellular‐therapies, allogeneic stem cell transplantation (allo‐HSCT) represents so far the only potentially curable treatment according to a longest follow-up in a setting of relapse/refractory Non-Hodgkin lymphoma (rrNHL). However, late effect (LE) issues of allo-HSCT in rrNHL seem to be underexplored. Here, we present the first analysis of a retrospective, multicenter, registry‐based analysis (Swiss Blood Stem Cell Transplantation and Cellular Therapy, SBST) of LE in patients who underwent allo‐HSCT for rrNHL in Switzerland.
Methods: We retrospectively collected data from 109 patients with all types of rrNHL who underwent allo-HSCT in 3 University Hospitals of Switzerland (Zurich, Basel and Geneva) between May 1997 and November 2021. The inclusion criteria was 2 years survival after allo-HSCT. The primary endpoint was the cumulative incidence (CI) of LE and secondary endpoint were overall survival (OS), relapse incidence (RI) and type of LE according to organ/systems involved.
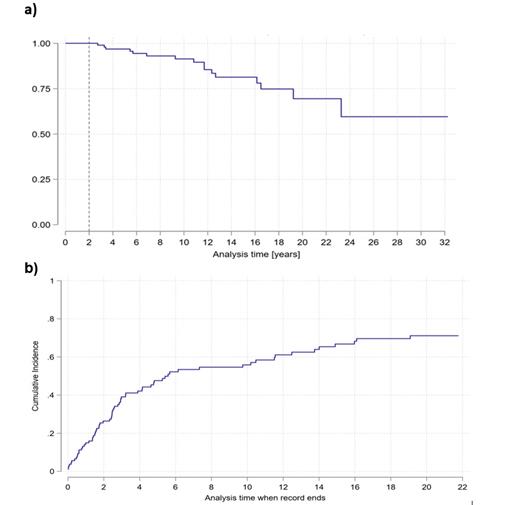
Results: median of follow-up was 6.5 years. Patients who survived 2 years after allo-HSCT presented 5 and 10 years OS of 94% [95% CI 86.3 - 97.5] and 86% [95% CI 75 - 92.4] respectively. RI and CI of LE were 18.3% and 44% at 5 years and 34.4% and 58% at 10 years post allo-HSCT. A total of 79/109 (72%) of patients experienced any type of LE during the follow-up period. Renal dysfunction (21%), secondary malignancies (18%), cardiovascular disease (18%), osteoporosis (18%) and thyroid dysfunction (17%) were the most frequently observed. CI of secondary malignancies was 6.1% and 20% at 5 and 10 years respectively.
Conclusions: Our analysis of a mixed-type rrNHL cohort confirms the efficacy of allo-HSCT in heavily pretreated/refractory patients, showing excellent OS at 5 and 10 years for those who survived 2 years. Both RI and LE present a competitive risk for survival benefit beyond 2 years after allo-HSCT. Secondary malignancies, renal and cardiovascular disease appear to be the most frequent types of LE, revealing the toxicity accumulated prior to allo-HSCT in the setting of rrNHL. These results should be interpreted with caution due to a retrospective nature of the analysis, the heterogeneity of the conditioning regimen and the NHL cohort.
Figure 1. a) Overall survival (KM-estimate) b) cumulative incidence of late effects
Disclosures
Chalandon:MSD: Honoraria, Other: travel support; Novartis: Honoraria, Other: travel support; Incyte: Honoraria, Other: travel support; BMS: Honoraria, Other: travel support; Pfizer: Honoraria; Abbvie: Honoraria, Other: travel support; Roche: Honoraria, Other: travel support; Jazz: Honoraria, Other: travel support, Speakers Bureau; Gilead: Honoraria, Other: travel support; Amgen: Honoraria, Other: travel support; Astra-Zeneca: Honoraria, Other: travel support; Servier: Honoraria; Sanofi: Other: travel support; Janssen: Other: travel support.