Introduction
CAR-T cell therapy, an FDA-approved breakthrough in hematologic malignancy treatment, has emerged as a game-changer through rigorous pivotal trials. Reportedly, women were underrepresented as authors in pivotal trials supporting approval of novel hematological drugs [1]. However, gender inequity in authorship across pivotal trials of FDA-approved novel CAR-T cell therapies remains unexamined. Thus, our study explores gender representation among authors in pivotal trials that led to the first-ever FDA approval of CAR-T cell therapies for hematological malignancies
Methods
Drugs@FDA portal was searched for novel CAR-T cell therapies approved between 2015 and 2022. Drug approval labels were searched to identify all pivotal trials listed in Section 14 under “Clinical Studies.” Only pivotal trials with corresponding trial publications were included in this study. The gender of trial authors was captured as binary (women and men) and was identified by using the Genderize software (Demografix ApS) or by verifying personal pronouns on authors' institutional profiles or other sources. Study-level estimates for the proportion of women authors were pooled using the DerSimonian and Laird random-effects meta-analysis of proportions model. Analyses were conducted with OpenMeta [Analyst] software version 10.12.
Results
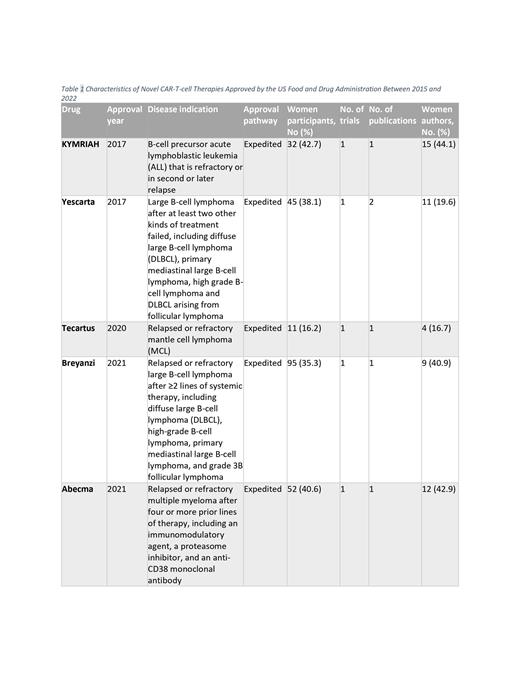
From 2017-2022, the FDA approved a total of 6 novel CAR-T cell therapies, corresponding to 6 trials and 7 trial publications (755 participants) that were included in the study (Table 1). One trial's findings were reported in two distinct publications. A total of 201 authors were screened, and 1 was excluded because of the inability to assign author gender. Genderize was used to identify the gender of 177 authors; 152 were further confirmed by checking personal pronouns. Of the 24 authors with a prediction probability estimate less than 90.0% on Genderize, author gender was identified for 23 by using personal pronouns listed in institutional profiles or other sources.
The median number of men and women authors of trial publications was 19 (IQR, 15-23) and 9 (IQR, 6-11), respectively. The overall proportion of women authors was 29.6% (95% CI, 20.5%-38.7%). The proportion of women authors varied significantly across the 7 trial publications (p-heterogeneity = 0.043). The proportion of women as first and senior authors was the same, estimating about 14.3% (95% CI, 0.0%-40.2%).
Conclusions
Our study highlights gender disparities in the authorship of pivotal efficacy trials supporting the FDA approval of novel CAR-T cell therapies. This underscores the urgent need for institutions to devise tailored policies that tackle the unique challenges faced by women in the medical field, while fostering an inclusive and supportive workplace environment that empowers women to achieve academic success.
Disclosures
Jaglal:Agios: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; SOBI: Membership on an entity's Board of Directors or advisory committees.