Introduction.Acquired hemophilia A (AH) is a rare, potentially life-threatening, bleeding disorder caused by the development of autoantibodies directed against coagulation factor VIII (FVIII). Incidence is 1-1.5 per million people per year with a morality rate as high as 22% and is especially dangerous in the elderly. Since autoantibodies rarely resolve spontaneously, initial management involves controlling acute bleeding episodes, and immunosuppressive treatment (IST) to eradicate the autoantibodies to restore hemostasis. There are no evidence-based guidelines for the management of AH and treatment decisions rely on the clinical judgement and expertise of the treating provider, which impacts overall health outcomes. There is a dearth of prospective, controlled clinical trials to guide management of AH and much of the reported data come from observational studies and extrapolation from studies on patients with congenital hemophilia complicated by inhibitors.
Methods.Retrospective chart review of AH patients treated by a single provider at Jackson Memorial Hospital from 2007 through May 2023 utilizing in-house medical record systems and supplemental external health records from transferring facilities. Partial remission (PR) was defined as FVIII activity >50 IU/dL and no active bleeding after stopping hemostatic drugs >24 hours. Complete remission (CR) was defined as PR plus negative inhibitor, prednisone tapered to <15 mg/day, and cessation of other IST.
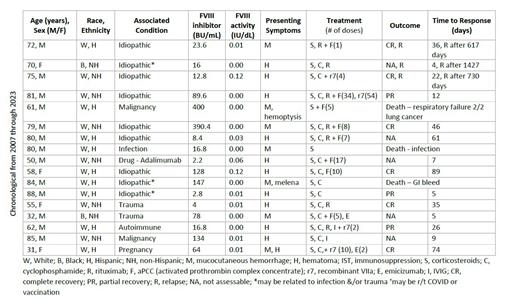
Results.We identified 17 patients with first episode of AH with laboratory evidence of acquired FVIII inhibitor (aFVIIIi) (see Table 1). The majority were male (76%), Hispanic (53%) and White (88%). The median age at diagnosis was 72 (range 31-88). While 65% of cases were idiopathic, other associated conditions included malignancy and trauma. The median aFVIIIi was 23.2 BU/mL (range 2.2-400) and median FVIII activity was 1% (range 0-12) at presentation. The most common presenting symptom was hematoma (59%) followed by mucocutaneous bleeding (41%). Hematuria was also seen frequently and initially attributed to another cause.
To control bleeding, 47% received activated prothrombin complex concentrate (aPCC), 17% received recombinant factor VIIa, and 12% received emicizumab. Emicizumab was initiated at an outside facility lacking bypassing agents for one patient, while the other had bleeding with neutropenic sepsis. All patients received IST with glucocorticoids; cyclophosphamide was used in 82%, rituximab in 41%, and IVIG in 12%. For patients with appropriate follow-up (median follow-up 707 days), 7/9 (78%) achieved a CR to IST and 2/9 (22%) developed PR. The median time to PR was 41 days and median time to CR was 168 days in patients who achieved those respective responses. Five patients (29%) responses were not assessable due to lack of appropriate follow-up. Three patients (17%) had a clinically evident relapse requiring retreatment at 617, 730, and 1427 days after initial response. Ultimately, three patients (17%) died or were discharged to hospice within a median of 8 days, but only one death was directly related to bleeding (which was prior to receiving any appropriate hemostatic agents).
Discussion.We report a single-provider experience on patients treated for AH, with focus on acute management and CR due to its impact on health outcomes. Our experience confirms the importance of initiating IST early in diagnosis and tapering as FVIII levels recover, finding the lowest effective dose to eradicate the inhibitor. It should be noted that bypassing agents are not always required if bleeding is not significant. While no one who received hemostatic agents died of hemorrhage, several patients required dozens of doses of these expensive medications. Recent reports using emicizumab off-label for AH suggest effective hemostasis in the acute phase, which was successful in two patients described here. Monitoring the response to emicizumab is more challenging due to lab interference with PTT and single-stage factor assays, and therefore requires bovine assays that are costly, untimely, and not widely available. A large proportion of patients referred from outside lacked appropriate follow-up after initial diagnosis for acute management. For optimal long-term outcomes, patients with AH should have ongoing follow up with periodic lab monitoring as relapses may occur several years after initial response in 17% or more of patients.
OffLabel Disclosure:
No relevant conflicts of interest to declare.
emicizumab is currently FDA approved for prophylaxis in individuals with hemophilia A with and without inhibitors. More recently it has been used off-label for acute bleed management in individuals with acquired hemophilia A.